
2492C 760 mmHg Partition coefficient n-octanolwater. C i e n o t e s.
560E-012 atm-m3mole Exper Database.
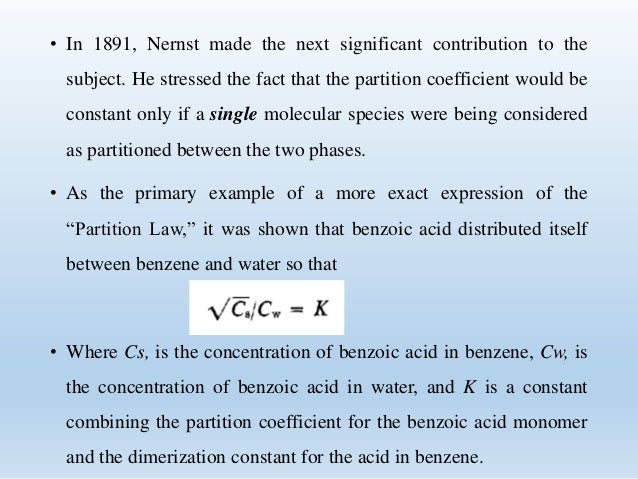
Benzoic acid partition coefficient. As the primary example of a more exact expression of the Partition Law it was shown that benzoic acid distributed itself between benzene and water so that Where Cs is the concentration of benzoic acid in benzene Cw is the concentration of benzoic acid in water and K is a constant combining the partition coefficient for the benzoic acid monomer and the dimerization constant. Partition coefficient Log Pow Benzoic acid. 188 Sodium benzoate.
Additional physical and chemical specifications Benzoic acid Organoleptic properties. Melting point. 122 C Boiling point.
2492C Flash point. Vapour pressure. 1321 gcm3 at 20C Viscosity.
-2269 Refractive index. Sodium benzoate Organoleptic. 249 C Food and Agriculture Organization of the United Nations Benzoic acid.
249 C OU Chemical Safety Data No longer updated More details. 249 C Alfa Aesar A14062 36230. 249 C Literature LabNetwork old LN00195619.
133 C 10 mmHg 2966803 C 760 mmHg FooDB FDB008739. 249 C SynQuest 2621-1-21. 249 C Sigma-Aldrich ALDRICH-491861 SAJ.
N-octanolwater No data available Autoignition Temperature Not applicable 570 C 1058 F. Benzoic acid Revision Date 23-Jan-2015 16. Other information Prepared By Regulatory Affairs Thermo Fisher Scientific Email.
Benzoic Acid Created by Global Safety Management Inc. 1224 deg C Solubilities. 34 gl 25C Boiling pointBoiling range.
2492C 760 mmHg Partition coefficient n-octanolwater. Not Determined Flash point closed cup. 570C Evaporation rate.
Estimated soil adsorption coefficients of 65 and 3101SRC obtained from o-chlorobenzoic acids water solubility 2087 mgL at 25 C2 and octanolwater partition coefficient 2053 respectively can be obtained using an appropriate regression equation. These values indicate that it will display moderate to high mobility in soil4. Estimated bioconcentration factors of 20 and 641SRC obtained from m-chlorobenzoic acids water solubility 450 mgL at 25 C2 and octanolwater partition coefficient 2683 respectively can be obtained using an appropriate regression equation.
These values indicate that it will not bioconcentrate in fish and aquatic organismsSRC. The acid dissociation constant for an acid is a direct consequence of the underlying thermodynamics of the dissociation reaction. The pK a value is directly proportional to the standard Gibbs free energy change for the reaction.
The value of the pK a changes with temperature and can be understood qualitatively based on Le Châteliers principle. When the reaction is. Benzoic acid associates in the oil phase and dissociates in the aqueous phase The species common to both the oil and water phases are the unassociated and undissociated benzoic acid molecules.
Extraction Partition coefficient is used to determine the efficiency with which one solvent can extract a compound from a second. Most efficient extraction results when a large number of. Characteristic partition coefficient with the following formula.
Partition coefficient solubility in ethersolubility in water. Carboxylic acid unknown options Part 1. Benzoic acid mp 123 or 2-chlorobenzoic acid mp 141 Amine unknown options Part 2.
4-chloroaniline mp 68-71 or ethyl 4-aminobenzoate mp 90 Neutral options same choices for both Part 1 and Part 2. YALKOWSKYSH DANNENFELSERRM 1992 ECOSAR Class Program ECOSAR v099h. Phenols-acid Salicylic Acid-acid Henrys Law Constant 25 deg C HENRYWIN v310.
142E-008 atm-m3mole Group Method. 560E-012 atm-m3mole Exper Database. 734E-09 atm-m3mole Henrys LC VPWSol estimate using EPI values.
1522E-009 atm-m3mole Log Octanol-Air. Benzoic acid 4-amino- ethyl ester 94-09-7 95 4. First-aid measures General Advice If symptoms persist call a physician.
Show this safety data sheet to the doctor in attendance. Eye Contact Rinse immediately with plenty of water also under the eyelids for at least 15 minutes. Immediately flush with plenty of water.
After initial flushing remove any contact lenses. Solubility Partition Coefficient Dissociation constant Hydrogen Bonding Ionization of Drug Redox Potential Complexation Surface activity Protein binding Isosterism 1. The solubility of a substance at a given temperature is defined as the concentration of the dissolved solute which is in equillibrium with the solid solute.
Solubility depends on the nature of solute and. Molar concentration also called molarity amount concentration or substance concentration is a measure of the concentration of a chemical species in particular of a solute in a solution in terms of amount of substance per unit volume of solution. In chemistry the most commonly used unit for molarity is the number of moles per liter having the unit symbol molL or moldm 3 in SI unit.
0100 mol dm-3 benzoic acid. A sharp fall between pH 11 and pH 75 Midpoint of steep slope at about pH 9. Partition coefficient K pc is the equilibrium constant which relates the concentration of a solute partitioned between two immiscible solvents at a particular temperature.
9 P a g e h t t p s. W w w. C i e n o t e s.
C o m Worked example. Acid catalyzed Acid chloride Acid dissociation constant Acid ionization constant. Benzoic acid Benzophenone.
Partition coefficient PBoR PCC Penicillin Pentane Pentapeptide Pentavalent carbon Pentene Pentet Pentose Peptide Peptide bond Peptidoglycan Per Peracetic acid Peracid Pericyclic reaction Period Periplanar Peroxide Peroxide effect Peroxyacetic acid Peroxyacid PET Pet ether PETE. Definition and Calculation Lanthanides. Definition Properties Applications of Coordination Compounds.
A mixture contains benzoic acid 4-chloroaniline and naphthalene. 3 Benzoic acid 2-hydroxy- 335-trimethylcyclohexyl ester Homosalate HOMOSALATE 118-56-9 204-260-8 10 02082021 4 2-Hydroxy-4-methoxybenzophenone Oxybenzone BENZOPHENONE-3 131-57-7 205-031-5 6 Reg EU 2017238 of 10 February 2017-date of application from September 2017 Not more than 05 to protect product formulation Contains Benzophenone-3 1 02082021 6 2. LogD refers the the wateroctanol partition coefficient at a specific pH normally pH 74.
For ionizable compounds acids and bases logD would be altered by pH because the distribution of. The constant is defined as the partition coefficient K. K C S C G.
Where C S is the concentration of the analyte in the sample after equilibrium the term C L may be used if the sample is a liquid and C G is the concentration of the analyte in the gas phase after equilibrium. The partition coefficient is lowered as the temperature increases and also varies with changes in the matrix. Benzoic acid Skin Irrit.
H315 H318 H372 H402. First aid measures 41 Description of first-aid measures General advice Show this material safety data sheet to the doctor in attendance. If inhaled After inhalation.
The acid dissociation constant. The partition coefficient depended on the pH of the aqueous phase. In the limiting case where the ionization is completely suppressed by pH for bases for example at high pH a distribution coefficient D can be defined mathematically for bases.
D B o B w 15 P B o B w BH w 16 Here B is the uncharged and BH the charged species. Academiaedu is a platform for academics to share research papers. This website uses cookies to help provide you with the best possible online experience.
Please read our Terms Conditions and Privacy Policy for information about. Canned Food Spoilage Low Acid pH 46 Acid pH 3740 to 46 High Acid pH. 15th May 2019 400-500 pm.
Basic principles of work in the analytical laboratory and Classical Methods of analysis Volumetric and Gravimetric Analysis DR.