Styrene consists of a benzene ring connected. Products Building Blocks Explorer Technical Documents Site Content Papers Genes.
Benzoic acid solid and 1-Propanol liquid Ester C.
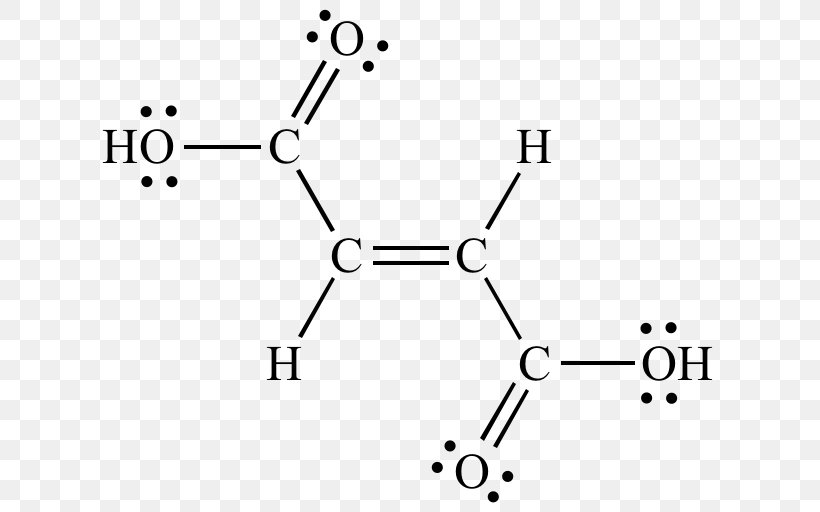
Benzoic acid lewis structure. 4-Hydroxybenzoic acid also known as p-hydroxybenzoic acid PHBA is a monohydroxybenzoic acid a phenolic derivative of benzoic acidIt is a white crystalline solid that is slightly soluble in water and chloroform but more soluble in polar organic solvents such as alcohols and acetone. 4-Hydroxybenzoic acid is primarily known as the basis for the preparation of its esters known as parabens. Octanoate salicylate valproic acid p-octyl- p-nitro- and p-chlorobenzoic acids were effective inhibitors of benzoic acid activation to benzoyl-CoA by mitochondrial extracts.
P-Aminobenzoic acid was much less effective. Of these compounds only salicylate and p-nitrobenzoic acid were not activated to their respective CoA esters. Salicylate p-chloro- and p-nitrobenzoic acids effectively.
An acid is a molecule or ion capable of either donating a proton ie hydrogen ion H known as a BrønstedLowry acid or capable of forming a covalent bond with an electron pair known as a Lewis acid. The first category of acids are the proton donors or BrønstedLowry acidsIn the special case of aqueous solutions proton donors form the hydronium ion H 3 O and are known as. 4-Nitrobenzoic acid C7H5NO4 CID 6108 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. For example if a solution of benzoic acid. The product of a Lewis acid-base reaction is a neutral dipolar or charged complex which may be a stable covalent molecule.
As shown at the top of the following drawing coordinate covalent bonding of a phosphorous Lewis base to a boron Lewis acid creates a complex in which the formal charge of boron is negative and that of phosphorous is. Nicotine also augments both glutamate release which facilitates the release of dopamine and γ-aminobutyric acid GABA release which inhibits dopamine release. 15 16 With long-term exposure to nicotine some nicotinic cholinergic receptors become desensitized but some do not.
As a result GABA-mediated inhibitory tone diminishes while glutamate-mediated excitation persists thereby. Contributing structure This resonance structure accounts for the selectivity Nitration of benzoic acid Organic Lecture Series 40 Activating-Deactivating Any resonance effect such as that of -Any resonance effect NH2 -OH and -OR that delocalizes the positive charge on the cation intermediate lowers the activation energy for its. In symmetrical acid anhydride carboxylic acid the only constituent used to form the compound so the prefix is carboxylic and suffix anhydride is used.
In asymmetrical acid anhydride two different carboxylic acids are used like the dehydration of benzoic acid and propanoic acid so the prefix is benzoic propanoic and anhydride is a suffix. Ethanoic acid forms ethanoic anhydride propanoic. Benzoic acid solid and 1-Propanol liquid Ester C.
Acetic acid liquid and 3-Methyl-1-butanol liquid Group 1 is assigned Esters A and B Group 2 is assigned Esters B and C Circle the 2 esters your group has been assigned. Group 3 is assigned Esters A and C These assignments will allow some replicate samples in case there are experimental errors. Glacial acetic acid and.
1212 Structure of the Carbonyl Group π Fig121 Orbital diagram for the formation of carbonyl group The carbon-oxygen double bond is polarised due to higher electronegativity of oxygen relative to carbon. Hence the carbonyl carbon is an electrophilic Lewis acid and carbonyl oxygen a nucleophilic Lewis base. Products Building Blocks Explorer Technical Documents Site Content Papers Genes.
Keyword MSDS Showing 1-2 of 2 results for MSDS within Products. Sigma-Aldrich SDS Subscription. Custom and intranet version quarterly.
Benzoic acid was eventually converted to the stable hydrocarbon benzene C 6 H 6 which also proved unreactive to common double bond transformations as shown below. For comparison reactions of cyclohexene a typical alkene with these reagents are also shown green box. As experimental evidence for a wide assortment of compounds was acquired those incorporating this exceptionally stable.
Products Building Blocks Explorer Technical Documents Site Content Papers Genes. Ships Today 39 Product Category. Antibodies 211 primary antibodies 211 bioactive small molecules 23 lipids 9 kits 3 Brand.
Sigma-Aldrich 249 Supelco 2 Biological Source. Benzoic Acid is is a white crystallize solid at room temperature. Benzoic acid and its salts sodium benzoate potassium benzoate etc are commonly used as preservatives in foods to prevent the growth of mold.
For more information of benzoic acid and its derivatives see the page on Carboxylic Acids. Add 3D Structure. Styrene consists of a benzene ring connected.
These phenolic acids contain C 6-C 3 structure and used as ancestor in the synthesis of lignins and other phenolics. By losing two carbon-atoms cinnamic acid transformed into benzoic acid and its derivatives. For the large scale production of phenolic acids biotechnological approaches have been used.
The cell and tissue cultures models are used to reveal the regulation of different. Benzoic acid Benzoic acid occurs as white needle shaped crystals found naturally in gum benzoin. Benzophenone Benzophenone is an aromatic compound with a fragrance like geranium.
Benzoapyrene Benzoapyrene is commonly found in cigarette smoke coal tar and fuel exhaust. P -Benzoquinone p -Benzoquinone can be highly toxic and fatal if swallowed inhaled or absorbed through the skin. The structure of a ZnCl 2 molecule is illustrated below.
Structure of Zinc Chloride Molecules. It is important to note that the zinc-chlorine bond in ZnCl 2 possesses some covalent characteristics which accounts for its low melting point and its solubility in ethereal solvents. The reaction between metallic zinc and hydrogen chloride gas yields the anhydrous form.
Chiral benzoic acid catalysts are reported that efficiently catalyse enantioselective 42 cycloadditions of acetals. The peculiar structure of the acids features covalently linked thiourea sites that stabilize the carboxylate conjugate bases via intramolecular hydrogen bond to the anionic site. This leads to the low p K a values of the acids compared benzoic acids with similar substitution.
Structure-based rationalization of selectivity of FTO inhibitors. Carboxymethylcarbamoyl-5-hydroxypyridin-2-ylaminobenzoic Acid 14f To a solution of the methyl ester 12f 359 mg 1 mmol in 14-dioxane 5 mL 1 M lithium hydroxide 50 mg 2 mmol was added. The mixture was stirred at room temperature for 24 h until consumption of the starting material and then acidified with.