This white solid is a commodity chemical used principally as a precursor to the polyester PET used to make clothing and plastic bottles. In higher dosages the increase in weight as well as the feed consumption was reduced.

Conventionally solutions are not 100 H₂SO₄ but are diluted to an extent with water and reported in weight percent wt.
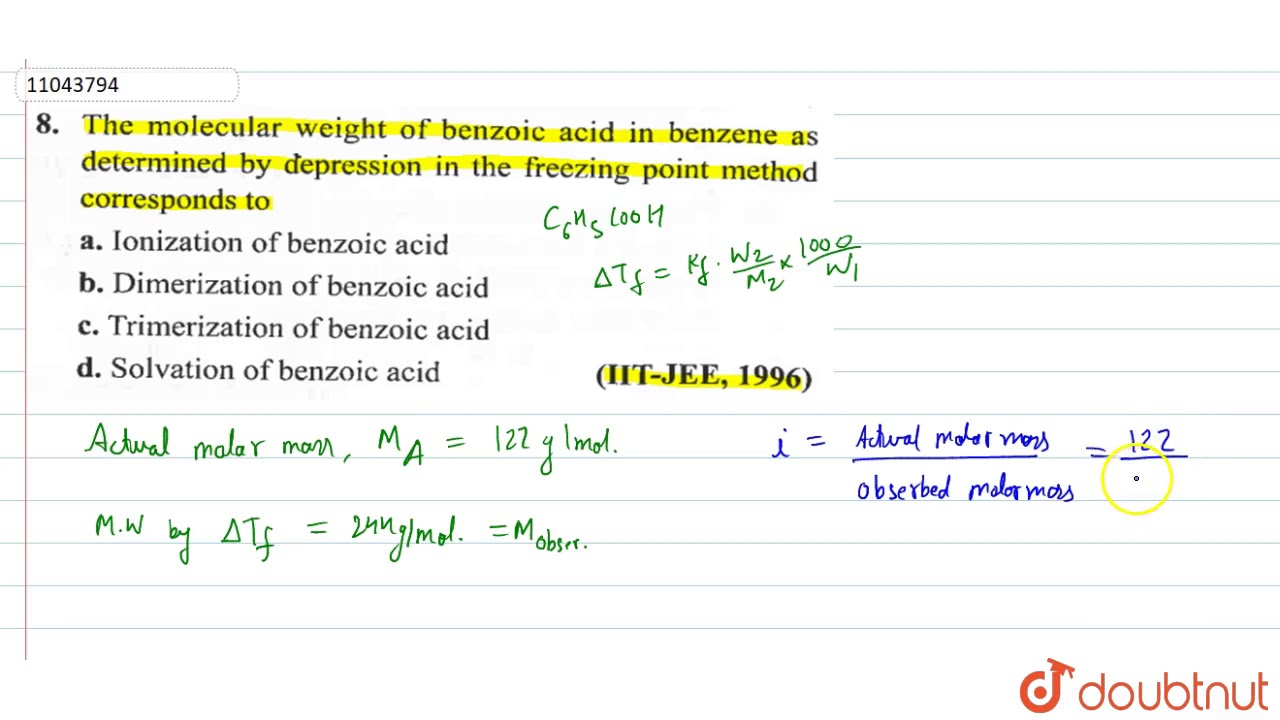
Benzoic acid formula weight. Benzoic acid is an organic compound which is described by the chemical formula C 6 H 5 COOH. It consists of a carboxyl group attached to a benzene ring. Therefore benzoic acid is said to be an aromatic carboxylic acid.
This compound exists as a crystalline colorless solid under normal conditions. The term benzoate refers to the esters and salts of C. Calculate the percent recovered using the following written formula and determine the melting point of your recrystallized benzoic acid.
Weight of benzoic acid obtained after recrystallization Recovered x100 Weight of benzoic acid before recrystallization Note. Submit product to the instructor in a properly labeled container. Weight of impure benzoic acid _____g.
3 Bring approximately 200 mL of water to a boil using the 250 mL round bottom flask fitted with a clamp as a handle using the heating mantle pages 150-152 OCLSM which is plugged into the variac. CHEM 2423 Recrystallization of Benzoic Acid Dr. Pahlavan 3 Example 1- The solubility of solid X in hot water 550 g100 ml at 100 oC is not very great and its solubility in cold water 053 g100ml at 0 oC is significantWhat would be the maximum theoretical percent.
Oxalic acid COOH2 - Oxalic acid is the smallest di-carboxylic acid with the chemical formula C2H2O4. Molecular Weight of oxalic acid is 9003 gmol. Visit BYJUS to understand the properties structure and uses of Oxalic acid C2H2O4 explained by Indias best teachers.
Weigh 10 g of benzoic acid recording the exact amount and place it into a 50 mL Erlenmeyer flask. Place 20 mL of distilled water into a second 50 mL Erlenmeyer flask. Add a boiling stick and using a hot plate heat the water to boiling.
Handle the hot flasks with a towel or tongs to prevent burning your fingers. Using a Pasteur pipette add 05-1 mL of the boiling solvent to the. Abietic acid C19H29COOH Acenaphthene C12H10 Acenaphthoquinone C12H6O2 Acenaphthylene C12H8 Acetaldehyde CH3CHO Acetanilide C8H9NO Acetic acid CH3COOH Acetone CH3COCH3 Acetonitrile CH3CN Acetophenone C8H8O Benzaldehyde C6H5CHO Benzene C6H6 Benzoic Acid C6H5COOH Benzyl Alcohol C7H8O Bromobenzene C6H5Br Bromomethane CH3Br Butanal.
Dimethocaine also known as DMC or larocaine is a compound with a stimulatory effectThis effect resembles that of cocaine although dimethocaine appears to be less potentJust like cocaine dimethocaine is addictive due to its stimulation of the reward pathway in the brain. C 8 H 8 O 2. O-toluic acid appears as pale yellow crystals or off-white flaky solid.
NTP 1992 CAMEO Chemicals. O-toluic acid is a methylbenzoic acid that is benzoic acid substituted by a methyl group at. Subchronic or Prechronic Exposure Fisher F344 rats were administered 4-nitrobenzoic acid at dosages of 0 025 05 1 2 and 4 by oral feed for 14 days.
No mortality was observed. In higher dosages the increase in weight as well as the feed consumption was reduced. Histologic changes were observed from 2 in seminal.
Analytical 4 ACS reagent 2 BioReagent 2 Technique. Available for Sale. Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within Products.
Hydrogen chloride solution. C 19 H 38 O 2. C 6 H 3 N 3.
Terephthalic acid is an organic compound with formula C 6 H 4 CO 2 H 2. This white solid is a commodity chemical used principally as a precursor to the polyester PET used to make clothing and plastic bottles. Several million tonnes are produced annually.
The common name is derived from the turpentine-producing tree Pistacia terebinthus and phthalic acid. Terephthalic acid was. Product NameButanoic acid 22-bishydroxymethyl-MDL NoMFCD00190088.
Biosynthesis of coenzyme A CoA from pantothenic acid vitamin B5 is an essential universal pathway in prokaryotes and eukaryotes. COASY is a bifunctional enzyme that catalyzes the 2 last steps in CoA synthesis. These activities are performed by 2 separate enzymes phosphopantetheine adenylyltransferase PPAT.
EC 2773 and dephospho-CoA kinase DPCK. EC 27124 in prokaryotes. These include lactic acid and lactates propionic acid and propionates citric acid acetic acid sorbic acid and sorbates benzoic acid and benzoates and methyl and propyl parabens benzoic acid derivatives.
Benzoates are most effective when undissociated. Therefore they require low pH values for activity 2540. The sodium salt of benzoate is used to improve solubility in foods.
Determine the empirical formula of the carboxylic acid. View Answer A 06800 g sample of carboxylic acid is burned in oxygen producing 1212 g of CO_2 and 04991 g of H_2O. The chemical formula for the acid is H₂SO₄.
Conventionally solutions are not 100 H₂SO₄ but are diluted to an extent with water and reported in weight percent wt. Common commercial strengths are 93 wt 96 wt and 985 wt. Many customers purchase diluted sulfuric acid typically at 70 wt or 50 wt strength.
The handling storage and reactivity considerations of diluted. A structural formula for the dimer of acetic acid is shown here. When the mouse pointer passes over the drawing an electron cloud diagram will appear.
The high boiling points of the amides and nitriles are due in large part to strong dipole attractions supplemented in some cases by hydrogen bonding. Acidity of Carboxylic Acids. The pK a s of some typical carboxylic acids are listed in.
Toluene C_6H_5CH_3 is oxidized by air under carefully controlled conditions to benzoic acid C_6H_5CO_2H which is used to prepare the food preservative sodium benzoate C6H5CO2Na.