The actual laboratory we will do is the recrystallization of benzoic acid from water using the temperature gradient method. She taught chemistry biology astronomy and physics at the high school college and graduate levels.
Benzoic acid is a good preservative on its own and combining it with sodium hydroxide helps it.
Benzoic acid dissolve in water. Ions are by definition polar so the general truism like dissolves like indicates the ions will then dissolve in water. In addition to temperature changes there are other ways to increase or decrease the water-solubility of benzoic acid. The addition of a strong acid decreases the ionization through the common ion effect.
Increasing the pH increases ionization. Add about 20 ml distilled water using a graduated cylinder to the flask and bring the mixture to the boiling point by heating on a hot plate while stirring the mixture and boiling gently to dissolve benzoic acid completely. Fig 1 benzoic acid solution Erlenmeyer flask hot plate Fig 1- Dissolving benzoic acid Remove the flask from the hot plate and examine the solution.
If there are. Benzoic acid is an organic acid first used in foods almost 100 years ago. It occurs naturally in prunes cinnamon and cloves.
The free acid form is poorly soluble in water and the sodium salt sodium benzoate is often used because of its greater solubility Fig. Benzoic acids antimicrobial activity is primarily against yeasts and. The actual laboratory we will do is the recrystallization of benzoic acid from water using the temperature gradient method.
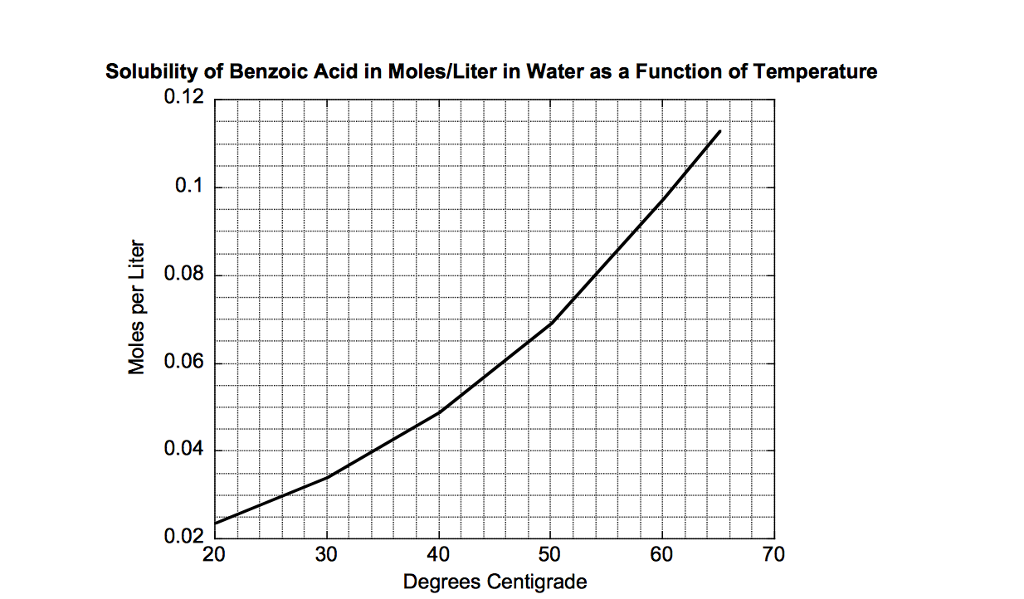
Benzoic acid is not very soluble in cold water but it is soluble in hot water. The purpose of this experiment is to learn the technique of recrystallization by purifying benzoic acid. CHEM 2423 Recrystallization of Benzoic Acid Dr.
Pahlavan 4 Experimental Procedures. By not allowing the benzoic acid to sit in the hot solvent long enough for it to dissolve. Because of this fact ethanol was used as the solvent in the first part of the experiment.
This caused a few problems most importantly was the fact that the solution was not recrystallizing once it began to cool after decolorization and filtration. This is the reason that water was added to the solution. Required to dissolve 10 g of benzoic acid at room temperature.
Calculate the volume of boiling water needed to dissolve 10 g of benzoic acid. Explain why a Büchner or Hirsh funnel is used to isolate the final crystallized product instead of stem funnel. Techniques and Transformations 4 4.
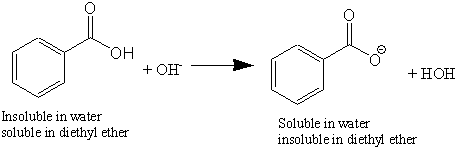
Explain when a mixture of solvents would be used to carry out a recrystallization. More specifically deprotonating benzoic acid forms a water-soluble carboxylate anion leaving the other organic by-products in the organic layer. Subsequent isolation and acidification of the aqueous layer re-precipitates the benzoic acid which can then be isolated by vacuum filtration.
The residual impurities in the organic layer will then be analyzed and identified using gas chromatography. This includes the melting point and solubilities in water and ether of benzoic acid and C O OH Benzoic Acid Naphthalene. Techniques and Transformations 2 naphthalene as well as the boiling point solubility in water and density of diethyl ether or tert-butyl methyl ether.
Before you come to the laboratory do the Pre-Lab assignments for this laboratory as assigned by your. Using a hot plate dissolve approximately 10 g of impure benzoic acid in 30 35 mL of hot water water at or near its bp in a 125 mL Erlenmeyer flask. If there is a residual amount of material that does not dissolve upon adding a small amount of additional solvent H2O do not continue to add more solvent.
It is important to use a MINIMUM amount of solvent in a. Treated with sodium bicarbonate and only benzoic acid becomes water-soluble. The other two compounds remain neutral still dissolved in the diethyl ether.
A stronger base sodium hydroxide is required to react with the less acidic 2-naphthol. The remaining two-component mixture in the ether layer can then be separated and 2-naphthol is then extracted from the remaining mixture. Note that 14.
Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 70. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt.
Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with. Its an odorless crystalline powder made by combining benzoic acid and sodium hydroxide. Benzoic acid is a good preservative on its own and combining it with sodium hydroxide helps it.
In asymmetrical acid anhydride two different carboxylic acids are used like the dehydration of benzoic acid and propanoic acid so the prefix is benzoic propanoic and anhydride is a suffix. Ethanoic acid forms ethanoic anhydride propanoic forms propanoic anhydride etc. It was first prepared by French chemist Charles Frederic Gerhardt in 1852 through heating potassium.
Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 70. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt.
Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with. Has covered chemistry for ThoughtCo and About Education since 2001 and other sciences since 2013. She taught chemistry biology astronomy and physics at the high school college and graduate levels.
Solution C 0l M citric acid. Dissolve 960 g AR citric acid in water and make up the solution to 500 mL in a measuring cylinder. Solution D 02 M disodium hydrogen phosphate.
Dissolve 1782 g Na2HPO42H2O in water and make up the solution to 500 mL in a measuring cylinder. Table 390 Standard buffer solutions. Water the glucose would mostly dissolve in the lower water phase phase layer and the naphthalene would mostly dissolve in the upper ether phase.
The two phases can then be physically separated using a pipet and placed into two separate tubes. The water and ether can then be evaporated to yield the separated solid compounds. In a nut shell this is an extraction.
1 Revised 032020. The pKa of benzoic acid is 42. The pKa of the conjugate acid of benzocaine is 25.
You dont need to track down any data on the indicator in part 1 everything you need is in this handout. 3 Calculate the number of mmol thats milimoles of benzoic acid benzocaine and fluorenone that you will be using in part 2. 4 Look up the densities and boiling points of the organic solvents you.
Any of a class of substances whose aqueous solutions are characterized by a sour taste the ability to turn blue litmus red and the ability to react with bases and certain metals to form salts. A substance that yields hydrogen ions when dissolved in water. A substance that can act as a proton donor.
Benzoic acid is more soluble in an organic solvent such as dichloromethane or diethyl ether and when shaken with this organic solvent in a separatory funnel will preferentially dissolve in the organic layer. The other reaction products including the magnesium bromide will remain in the aqueous layer clearly showing that separation based on solubility is achieved. This process known as.
Use a procedure that will oxidize the benzaldehyde to benzoic acid. You must use a 250-mL round bottom flask for this oxidation reaction. Perform a recrystallization on the benzoic acid you have prepared.
Remember recrystallization is when you take a solid chemical and without any chemical modification dissolve it in a solvent to. The pH of a simple solution of an acid compound in water is determined by the dilution of the compound and the compounds K a. Examples in organic acids include formic acid HCOOH acetic acid CH 3 COOH and benzoic acid C 6 H 5 COOH.
Polyprotic acids also known as polybasic acids are able to donate more than one proton per acid molecule in contrast to monoprotic. The actual laboratory we will do is the recrystallization of benzoic acid from water using the temperature gradient method and pH method. Benzoic acid is not very soluble in cold water but it is soluble in hot water.
The purpose of this experiment is to learn the technique of recrystallization by. Thus the benzoic acid could be separated from the neutral compound by extraction with either aqueous sodium bicarbonate or aqueous sodium hydroxide solution. We have chosen to extract the acidic compound into the aqueous layer using sodium hydroxide.
Outline the steps of the following procedure. Place 3 g of the mixture in a 50 mL Erlenmeyer flask and add 30 mL of diethyl ether. Chew or dissolve in mouth.
Adults and children 12 years and over. 2 tablets every 12 to 1 hour as needed. Do not exceed 8 doses 16 tablets in 24 hours.
Avoid excessive heat over 104ºF 40ºC Pepto-Bismol Liquid. Each 30 mL dose cup contains. Magnesium 25 mg sodium 8 mg.