
The hydrolysis of an ester in the presence of a base is called _____. Introduction of substituents into the aromatic ring makes the benzylic system prone to further deblocking methods.
Around 1832 benzonitrile the nitrile of benzoic acid.
Benzoic acid and benzyl alcohol polarity. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is a useful solvent due to its polarity low toxicity and. C 6 H 5 MgBr with formaldehyde and the Cannizzaro reaction of benzaldehyde also give benzyl alcohol.
The latter also gives benzoic acid an example of an organic disproportionation reaction. Like most alcohols it reacts with carboxylic acids. PubMedFinal report on the safety assessment of Benzyl Alcohol Benzoic Acid and Sodium Benzoate.
PubMedCompetitive binding of aroma compounds by beta-cyclodextrin. PubMedInhibition of malonaldehyde formation from blood plasma oxidation by aroma extracts and aroma components isolated from clove and eucalyptus. PubMedFree and bound volatile composition and characterization.
Benzyl ethers are stable to bases and weak acids as used to cleave acetals. Their removal can. For instance ruthenium dioxidesodium periodate to furnish base labile benzoic acid esters Scheme 34.
Introduction of substituents into the aromatic ring makes the benzylic system prone to further deblocking methods. Thus o-nitrobenzyl ethers available from o. Benzoic acid A A - Benzyl alcohol A A 80C -Bismuth carbonate Satd.
A A - Borax A A - Boric acid A A - Brine Satd. A A - Bromine liquid 100 D - - Bromine water a C - - Butyl acetate 100 C C - Butyl alcohol 100 A A - Calcium carbonate Satd. A A - Calcium chlorate Satd.
A A - Calcium chloride 50 A A - Calcium hydroxide A A - Calcium. Search Protein Data Bank entries by either ligand protein binding site and or binding mode information. A Benzyl alcohol b Cyclohexanol c Phenol d m-Chlorophenol Solution.
D Alcohols are less acidic than phenol. Further electron withdrawing group like Cl increases the acidity of phenol therefore m-chlorophenol is most. Mark the correct order of decreasing acid strength of the following Solution.
The acid found in vinegar is A. The acid found in ants is A. The hydrolysis of an ester in the presence of a base is called _____.
Biodegradable And Non Biodegradable Polymers. Biodegradable And Non Biodegradable. Biological Importance Of Calcium And Magnesium.
A Give a balanced equation use structures for the reaction that occurs when the mixture of benzoic acid 4-tert-butylphenol and 14-dimethoxybenzene is reacted with aqueous NaHCO3. Please use this book to increase your knowledge for the laboratory pratictioner. Around 1832 benzonitrile the nitrile of benzoic acid.
Suggesting it to be an ether of propionic alcohol and hydrocyanic acid. The synthesis of benzonitrile by Hermann Fehling in 1844 by heating ammonium benzoate was the first method yielding enough of the substance for chemical research. Fehling determined the structure by comparing his results to the already known synthesis of hydrogen.
Academiaedu is a platform for academics to share research papers. The free acid forms are water-soluble giving clear and tacky films. Solution rheology can be modified by the addition of salts and bases.
Gantrez MS-955 is a mixed salt of sodiumcalcium and is supplied as a free-flowing powder. The copolymer is soluble in water and produces solutions with high viscosity. Ethyl isopropyl and n-butyl half esters are produced by opening up the anhydride in.
Benzoic acid was reduced to benzaldehyde in 25 yield in the presence of. Benzyl alcohol was reduced to diene 33 in 26 yield under our conditions without losing the benzylic hydroxy group. This diene was previously prepared in two steps.
N-Methylindole was converted to 34 or 35 without or with t-butanol in 65 or 56 yield whereas other methods afforded only one of the two products 13. Sep 23 2020 A compound X is formed by the reaction of a carboxylic acid C 2 H 4 O 2 and an alcohol in presence of a few drops of H 2 SO 4. The organic compound is heated strongly with excess of CuO Cupic Oxide in an atmosphere of CO 2 where free nitrogen CO 2 and H 2 O are obtained.
Nov 18 2013 If not enough acid was added to protonate all the product some will stay in the aqueous. Nomenclature Of Substituted Benzene Compounds. Phenol Physical Chemical Properties.
Aldehydes Ketones and Carboxylic Acids. The 4- carboxylic acid group of 138 was important to maintain RORc inverse activity. Introduction of a hydroxyl at the C-3 of the 4-benzoic acid moiety of 138 resulted in compound 140 with improvement in RORc inverse agonist potency EC 50 2 nM over 139 but also with increased PPARγ affinity IC 50 650 nM.
Benzoic acid 218 182 70 98 Oleic acid 156 143 31 143 Stearic acid. This trend toward less polarity with increasing molecular weight within a class also accounts for the observation that lower molecular weight solvents are often stronger than higher molecular weight solvents of the same class although determinations of solvent strength must really be made in terms of the solvents. Polymer_handbookpdf - Free ebook download as PDF File pdf Text File txt or read book online for free.
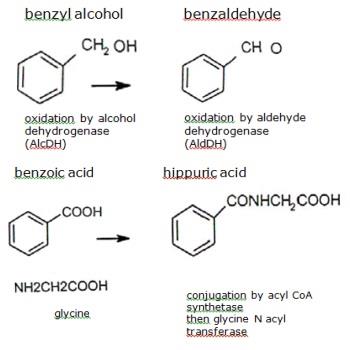
COMPOUND OTHER NAMES SOLVENT WAVELENGTH nm APPLICATIONS 25-dihydroxy benzoic acid DHB Gentisic acid Acetonitrile water methanol acetone chloroform 337 355 266 Peptides nucleotides oligosaccharides oligonucleotides 35-dimethoxy-4- hydroxycinnamic acid Sinapic acid sinapinic acid. SA Acetonitrile water acetone chloroform 337 355 266 Peptides proteins lipids. Iv Benzyl alcohol to benzoic acid.
V Dehydration of propan-2-ol to propene. Vi Butan-2-one to butan-2-ol Ans. I Acidified K2 Cr2 O7 or KMnO4 ii Pyridinium chlorochromate PCC in CH2 Cl2 or pyridinium dichromate PDC in CH2 Cl2.
Iii Aqueous Br2 ie. Br2H2O iv Acidified or alkaline KMnO4 followed by hydrolysis with dil. H2 SO4 at 443 K.
Vi NaBH4 or NiH2. Its quite an experience hearing the sound of your voice carrying out to a over 100 first year. Thank you for your participation.
Your assessment is very important for improving the workof artificial intelligence which forms the content of this project. LAMMPS Publications This page lists papers that cite LAMMPS via the original 1995 J Comp Phys paper discussed here which includes a discussion of the basic parallel algorithms in LAMMPSPapers that describe later algorithmic development in LAMMPS are also listed here. This list is generated from the Thomson Reuters Web of Science bibliographic database.