Chlorophyllin b acid form. Benzoic acid benzenecarboxylic acid Benzoic acid is the older name but is still in common use - its a lot easier to say and write than the modern alternative.
化学的特性 Benzoic acid pronunciation.
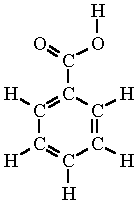
Benzenecarboxylic acid formula. 14-Benzenedicarboxylate - chemical information properties structures articles patents and more chemical data. Benzoic acid b ɛ n ˈ z oʊ. ɪ k is a white or colorless solid with the formula C 6 H 5 CO 2 H.
It is the simplest aromatic carboxylic acidThe name is derived from gum benzoin which was for a long time its only sourceBenzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. Salts of benzoic acid are used as food. Trimesic acid also known as benzene-135-tricarboxylic acid is an organic compound with the formula C 6 H 3 CO 2 H 3It is one of three isomers of benzenetricarboxylic acid.
A colorless solid trimesic acid has some commercial value as a precursor to some plasticizers. Trimesic acid can be combined with para-hydroxypyridine to make a water-based gel stable up to 95 C. Benzoic acid benzenecarboxylic acid has the -COOH group attached to a benzene ring.
Its physical and chemical properties are in line with those of any other carboxylic acid of a similar size so I havent felt it necessary to write about it separately. If you are interested in amino acids you could follow this link to the amino acids and proteins menu. Examples of carboxylic acids.
Benzenecarboxylic acid Benzoic acid 1-chloromethyl ethanoate. Esters are known for their distinctive odors and are commonly used for food aroma and fragrances. The general formula of an ester is RCOOR.
Esters are formed through reactions between an. Chlorophyllin a acid form. C 34 H 34 N 4 O 5.
Chlorophyllin b acid form. C 34 H 32 N 4 O 6. Each may be increased by 18 daltons if the cyclopentenyl ring is cleaved.
Content of total chlorophyllins is not less than 95 of the sample dried at ca. 100 C for 1 hour. 405 nm in aqueous.
Fisher Scientific 1 Reagent Lane Fair Lawn NJ 07410 For information call. 201-796-7100 For CHEMTREC assistance call. 800-424-9300 For International CHEMTREC assistance call.
Molecular Formula C7 H6 O2 Molecular Weight 12212 10. Stability and reactivity Reactive Hazard None known based on information available Stability Stable under normal conditions. Conditions to Avoid Incompatible products.
Incompatible Materials Strong acids Strong. Chemical properties of benzoic acid. Chemical properties of benzoic acid.
PKa values of Carboxylic acids Alcohols Phenols Amines. Post navigation Entropy Calculation for Ideal Gas Elements General Physical Properties. Atomic Size Melting point Boiling point.
Benzoic acid benzenecarboxylic acid Benzoic acid is the older name but is still in common use - its a lot easier to say and write than the modern alternative. Whatever you call it it has a carboxylic acid group -COOH attached to the benzene ring. Cases where the name is based on phenyl.
Remember that the phenyl group is a benzene ring minus a hydrogen atom - C 6 H 5. If you draw a. 249 C Alfa Aesar.
249 C Food and Agriculture Organization of the United Nations Benzoic acid. 249 C OU Chemical Safety Data No longer updated More details. 249 C Alfa Aesar A14062 36230.
249 C Literature LabNetwork old LN00195619 133 C 10 mmHg 2966803 C 760 mmHg FooDB FDB008739 249 C SynQuest 2621-1-21. Carboxylic acids can generally be expressed via the formula R-COOH. Carboxylic acids have a polar nature.
They can also participate in hydrogen bonding owing to their hydrogen bond accepting nature of the CO group and the hydrogen bond donating nature of the O-H bond. They generally have higher boiling points when compared to water and tend to form stable dimers. Carboxylic acids play an.
This should be done to give the lowest possible numbers to the substituents. When two or more. The general formula of a carboxylic acid is RCOOH with R referring to the rest of the molecule.
Paraffinic acid Alkanoic acid. A carboxylic acid where the R is an alkyl group. A carboxylic acid with two acid groups -COOH.
A carboxylic acid where the acid group is substituted to one carbon of a benzene ring. A Benzoic acid is the chemical benzenecarboxylic acid C 7 H 6 O 2 occurring in nature in free and combined forms. Among the foods in which benzoic acid occurs naturally are cranberries prunes plums cinnamon ripe cloves and most berries.
Benzoic acid is manufactured by treating molten phthalic anhydride with steam in the presence of a zinc oxide catalyst by the hydrolysis of. Academiaedu is a platform for academics to share research papers. Benzoic acid is a colorless crystalline solid also known as benzenecarboxylic acid.
It is the simplest aromatic carboxylic acid with a carboxyl group -COOH bonded directly to the benzene ring. It is found naturally in the benzoin resin of a number of plants. 化学的特性 Benzoic acid pronunciation.
C 7 H 6 O 2 or C 6 H 5 COOH is a colorless crystalline solid and a simple aromatic. Academiaedu is a platform for academics to share research papers.