
3 norcaradiene could be They are also used as a solvent and as a medium for various polymerization reactions. The Gutmann Acceptor AN and Donor number DN are measures of the strength of solvents as Lewis acids or bases.
It has a role as a flavouring agent a fragrance an odorant receptor agonist a plant metabolite an EC 3551 nitrilase inhibitor and an EC 3113 triacylglycerol lipase inhibitor.
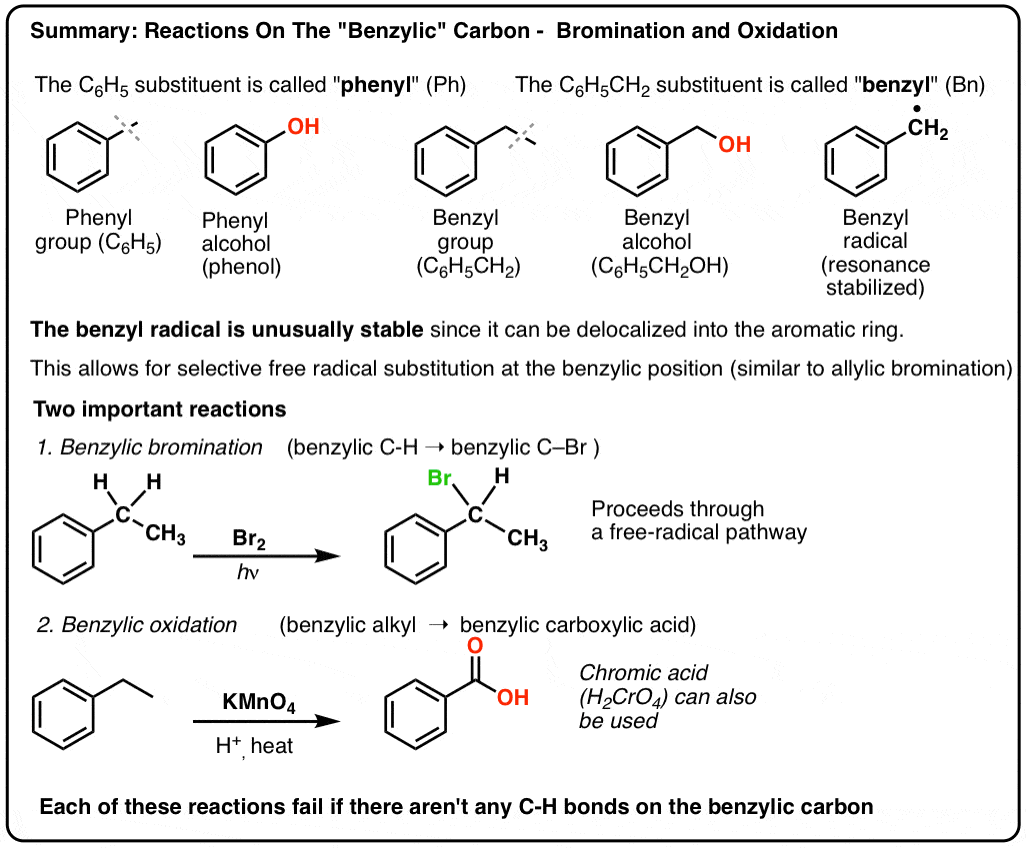
Benzene vs benzyl alcohol. Benzyl features a benzene ring attached to a CH 2 group. In IUPAC nomenclature the prefix benzyl refers to a C 6 H 5 CH. Benzyl is commonly used in organic synthesis as a robust protecting group for alcohols and carboxylic acids.
Treatment of alcohol with a strong base such as powdered potassium hydroxide or sodium hydride and benzyl halide BnCl or BnBr. The benzyl group abbv. Bn similar to the phenyl group is formed by manipulating the benzene ring.
In the case of the benzyl group. Whereas an OH group attached to a benzyl group would simply be called benzyl alcohol. Additionally other substituents can attach on the benzene ring in the presence of the benzyl group.
An example of this can be seen. Benzyl iodide is an organic compound with the chemical formula C 7 H 7 I. The compound consists of a benzene ring with an attached iodidemethyl group.
The substance is an alkyl halide and is a constitutional isomer of the iodotoluenes. 4-Bromophenyl phenyl ether Benzene 1-bromo-4-phenoxy- Brucine Strychnidin-10-one 23-dimethoxy–Poison B 1-Butanol n-Butyl alcohol 2-Butanone peroxide Methyl ethyl ketone peroxide-Toxic Butyl benzyl phthalate 12-Benzenedicarboxylic acid butyl phenylmethyl ester. Two Important Reaction Patterns.
Ortho- Para-Directors and Meta-Directors Its one thing to learn about electrophilic aromatic substitution reactions of benzene itself. But once you move beyond benzene thats when things start getting really interesting. Today well describe the two main patterns by which substituents direct electrophilic aromatic substitution.
Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. Acetone - Thermophysical Properties - Chemical physical and thermal properties of acetone also called 2-propanone dimethyl ketone and pyroacetic acid. Benzene - Dynamic and Kinematic Viscosity vs.
T-butylbenzene is much more p-directing than toluene 795 para for t-butylbenzene vs. 40 para for toluene which is likely due to sterics ortho approach is blocked by the bulkier t-butyl group. The transmission of polar effects through aromatic systems.
The nitration of benzyl derivatives J. Lignin the only biomass component rich in aromatic benzene ring structures is currently underutilized for low-value heat or treated as process waste because of its high chemical resistance. In this review a four-step pathway of pretreatment depolymerization hydrodeoxygenation HDO and alkylation to convert lignin into jet-fuel-range aromatic hydrocarbons and cycloalkanes is explored.
Hazardous waste is waste that poses a severe threat to human health or the environment if improperly disposed of. According to the EPA a substance is a hazardous waste if it appears on specific lists of hazardous waste or exhibits the established characteristics of hazardous waste. Hazardous waste is regulated under the Resource Conservation and Recovery Act RCRA.
Some common fluids and their refractive index. Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. Absorbed Solar Radiation - Solar radiation absorbed by various materials.
Acetone - Thermophysical Properties - Chemical physical and thermal properties of acetone also called 2-propanone. NMR MS peaks are narrower than NMR peaks MS is much more 104 x. Alcohol Fragmentation Molecular ion strength depends on substitution primary alcohol weak M secondary alcohol VERY weak M tertiary alcohol M usually absent Dehydration fragmentation thermal vs.
14-dehydration of M Loss of alkyl group largest R group lost as radical. C H 3 C H 2 C H 2. Spic and Span Disinfecting All-Purpose Spray Glass Cleaner.
15X Concentrate Closed Loop 3-05 21 gal. 2 units of 1 GAL each More Details. 37000325352 - Less Details.
Resin Comparison and Chemical Compatibility Chart Based on Nalgene Plastic Containers Look up chemical compatibility for major resins. LDPE - low density polyethylene. HDPE - high density polyethylene.
PP - Polypropylene and polypropylene copolymer. PET PETG and PETE - polyethylene terephthalate. Teflon type resins - PTFE Polytetrafluoroethylene FEP.
Our purpose is to solve the toughest problems in life science by collaborating with the global scientific community and through that we aim to accelerate access to better health for people everywhere. This sort of process is difficult to use in the. 3 M THF in water gave a 5248 mixture of alcoholether.
3 norcaradiene could be They are also used as a solvent and as a medium for various polymerization reactions. Geometrical or 1 2 3 C4 H3 cis-trans isomers of but-2-ene are represented as. H2C CH CH2 I But-1-ene Chain of 4 carbon atoms 12 3 4 Z 2 3 hydroxyprop 1 en 1 yl.
Geogard ECT Benzyl Alcohol Salicylic Acid Glycerin Sorbic Acid is a natural broad spectrum Ecocert approved preservative. Its very common among natural products especially in acid exfoliants due to the presence of salicylic acid which has a double effect it acts as both exfoliating agent and preservative. The producer states Geogard ECT is water soluble and it definitely is.
Common alcohol based VOCs include ethyl alcohol isopropyl alcohol benzyl alcohol while acetone methacrylates methyl or ethyl ethyl acetate and methyl ethyl ketone are ketone based VOCs. Alcohols mainly ethanol increase the formation of aldehydes by secondary reactions which cause throat irritation shortness of breath eye irritation and chest tightness Zhu and Wu 2015. The Gutmann Acceptor AN and Donor number DN are measures of the strength of solvents as Lewis acids or bases.
The Acceptor Number is based on the 31 P-NMR chemical shift of triethylphosphine oxide in the solvent. The Donor Number is based on the heat of reaction between the compound dissolved in CH 2 ClCH 2 Cl and SbCl 5 is therefore a solute rather than a solvent scale. Boiling points of some important liquids are water 100 C Ethyl alcohol 78.
60 We first find the number of moles of gas using the ideal gas equation. Calculate the vapour pressure of water at 293 K when 25 g of glucose is dissolved in 450 g water. Boiling points are listed below celsius.
Boiling point C When talking about boiling point we are typically referring to the normal boiling. 3 20 C vs air vapor pressure 73 mm Hg 20 C refractive index n 20D 13720lit FEMA 2414 ETHYL ACETATE Flash point. 26 F storage temp.
Store at 2C to 25C. Solubility Miscible with ethanol acetone diethyl ether and benzene. Pka 16-18at 25 form Liquid Specific Gravity 0902 2020 color APHA.
Il est utilisé comme conservateur alimentaire et est naturellement présent dans certaines plantes. Cest par exemple lun des principaux constituants de la gomme benjoin utilisée dans des encens dans les églises de Russie et dautres communautés orthodoxesBien quétant un acide faible lacide benzoïque nest que peu soluble dans leau du fait de la présence du cycle. PTFE Chemical Compatibility Chart.
Polytetrafluoroethylene is very non-reactive and ideal for use with most chemicals. Review the chemical compatibility of Teflon and PTFE with various chemicals solvents alcohols and other products in the cart below. Benzaldehyde is an arenecarbaldehyde that consists of benzene bearing a single formyl substituent.
The simplest aromatic aldehyde and parent of the class of benzaldehydes. It has a role as a flavouring agent a fragrance an odorant receptor agonist a plant metabolite an EC 3551 nitrilase inhibitor and an EC 3113 triacylglycerol lipase inhibitor. Tritylation of benzyl alcohol — 4-4-methyl-1-piperidinylpyridine MPP as catalyst 3.
A 1-methylcyclohexanol H2SO4 heat b product of a H2 Pt Solution for Predict the major products including stereochemistry when cis-3-methylcyclohexanol reacts with the following reagents. Predict the solubility of eplerenone and tiotropium bromide in wate 9.