Editorauthors are masked to the peer review process and editorial decision-making of their own work and are not able to access this work in the online manuscript submission system. Cetylpyridinium chloride is a pyridinium salt that has N-hexadecylpyridinium as the cation and chloride as the anion.
Cetylpyridinium chloride CPC is a cationic quaternary ammonium compound used in some types of mouthwashes toothpastes lozenges throat sprays breath sprays and nasal spraysIt is an antiseptic that kills bacteria and other microorganisms.
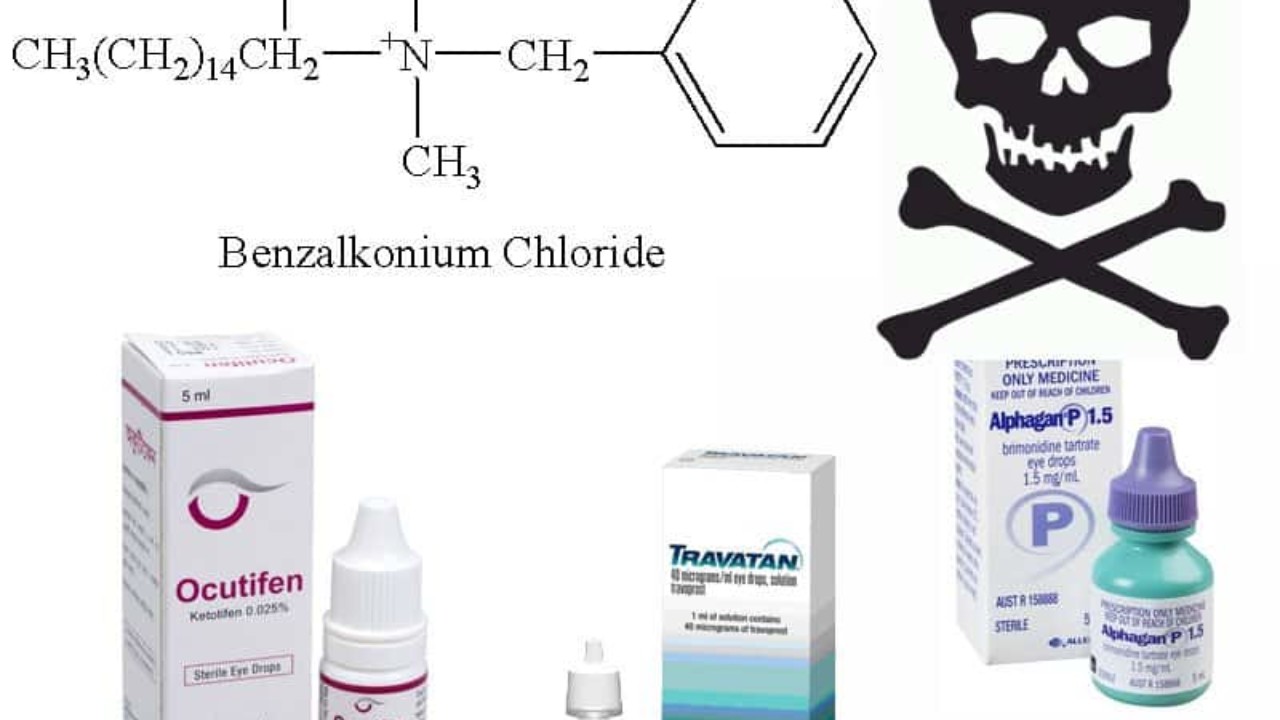
Benzalkonium chloride safety. Benzalkonium chloride was deferred from further rulemaking in the 2019 FDA Final Rule on safety and effectiveness of consumer hand sanitizers to allow for the ongoing study and submission of additional safety and effectiveness data necessary to make a determination on whether it met these criteria for use in OTC hand sanitizers but the agency indicated it did not intend to take action to. In this study we systematically examine the effect of the biocide benzalkonium chloride. Safety and effectiveness of health care antiseptics.
Topical antimicrobial drug products for over-the-counter human use. Here we have identified a distinct and broad-acting mechanism by which BAC which is not currently restricted. SAFETY DATA SHEET Creation Date 14-Jul-2014 Revision Date 19-Jan-2018 Revision Number 4 1.
Identification Product Name Benzalkonium chloride 50 wt aqueous solution Cat No. AC263820025 Synonyms Alkyl-benzyl-dimethylammonium chloride Recommended Use Laboratory chemicals. Uses advised against Food drug pesticide or biocidal product use.
Bibliographie en Bernstein IL Is the use of benzalkonium chloride as a preservative for nasal formulations a safety concern. J Allergy Clin Immunol. En Graf P Adverse effects of benzalkonium chloride on the nasal mucosa.
Allergic rhinitis and rhinitis medicamentosa Clin Ther. En Graf P Hallen H Juto JE Benzalkonium chloride. Cetylpyridinium chloride CPC is a cationic quaternary ammonium compound used in some types of mouthwashes toothpastes lozenges throat sprays breath sprays and nasal spraysIt is an antiseptic that kills bacteria and other microorganisms.
It has been shown to be effective in preventing dental plaque and reducing gingivitis. It has also been used as an ingredient in certain pesticides. Under this pesticide reregistration program EPA examines newer health and safety data for pesticide active ingredients initially registered before November 1 1984 and determines whether the use of the pesticide does not pose unreasonable risk in accordance to newer safety standards such as those described in the Food Quality Protection Act of 1996.
Alkyl dimethyl benzyl ammonium chloride. The preservative in ZERVIATE benzalkonium chloride may be absorbed by soft contact lenses. Lenses may be reinserted 10 minutes following administration of ZERVIATE.
The most commonly reported adverse reactions occurred in approximately 17 of patients treated with either ZERVIATE or vehicle. These reactions were ocular hyperemia instillation site pain and visual. Cetylpyridinium chloride is a pyridinium salt that has N-hexadecylpyridinium as the cation and chloride as the anion.
It has antiseptic properties and is used in solutions or lozenges for the treatment of minor infections of the mouth and throat. BZK Antiseptic Wipes Benzalkonium Chloride BZK Antiseptic Wipes are an alcohol free no. Hudson AddiPak Sodium Chloride 09 Unit Dose Saline - 3 ml.
Hudson AddiPak Sodium Chloride 09 Unit Dose Saline - 3 ml Hudson RCI - AddiPak Unit Dose Saline vi. Monoject Sodium Chloride 09 Prefilled IV. A safety data sheet material safety data sheet or product safety data sheet are documents that list information relating to occupational safety and health for the use of various substances and products.
SDSs are a widely used system for cataloging information on chemicals chemical compounds and chemical mixtures. International chemical safety cards icscs international chemical safety cards icscs introduction. Hydrogen icsc 12-dibromo-3-chloropropane icsc lead chromate icsc methyl isocyanate icsc paraquat dichloride icsc parathion icsc phosgene icsc tetraethyl lead icsc acetaldehyde icsc allyl chloride icsc aniline icsc antimony trioxide icsc arsenic.
The preservative in PROLENSA benzalkonium chloride may be absorbed by soft contact lenses. Lenses may be reinserted after 10 minutes following administration of PROLENSA The most commonly reported adverse reactions in 3-8 of patients were anterior chamber inflammation foreign body sensation eye pain photophobia and blurred vision. Benzalkonium chloride has been reported to cause eye irritation symptoms of dry eyes and may affect the tear film and corneal surface.
Should be used with caution in dry eye patients and in patients where the cornea may be compromised. Patients should be monitored in case of prolonged use. Nasal Zero Benzalkonium chloride may cause irritation or swelling.
Benzalkonium chloride may be absorbed by. Patients who wear soft contact lenses and. Whose eyes are not red should be instructed to wait at least ten minutes after instilling.
PATADAY olopatadine hydrochloride ophthalmic solution 02 before they insert their contact lenses. 6 ADVERSE REACTIONS. Symptoms similar to cold syndrome and pharyngitis were reported at.
Customer Support Contact Us FAQ Safety Data Sheets SDS Certificates COACOO Quality Regulatory Calculators Apps Webinars. Orders Quick Order Custom Products Commerce Solutions. Company About Us Responsibility Events Press Releases Programs Careers Offices.
We are a leading supplier to the global Life Science industry with. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. The safety and effectiveness of brimonidine tartrate have not been studied in children below the age of 2 years.
In a well-controlled clinical study conducted in pediatric glaucoma patients ages 2 to 7 years the most commonly observed adverse reactions with. ALPHAGAN 02 dosed three times daily were somnolence 50-83 in patients ages 2 to 6 years and decreased alertness. EUFLEXXA should not be administered through a needle previously used with medical solutions containing benzalkonium chloride.
Do not use skin disinfectants for skin preparation that contain quaternary ammonium salts. Do not inject intravascularly due to potential for systemic adverse events. The safety and effectiveness of injection in conjunction with other intra-articular injectables or.
The Journal of Endodontics the official journal of the American Association of Endodontists publishes scientific articles case reports and comparison studies evaluating materials and methods of pulp conservation and endodontic treatmentEndodontists and general dentists can learn about new concepts in root canal treatment and the latest advances in techniques and. Azarga contains benzalkonium chloride which is known to discolour soft contact lenses. Therefore care should be taken by people who wear soft contact lenses.
Why has Azarga been approved. The Committee for Medicinal Products for Human Use CHMP noted that combining the two active substances in Azarga simplifies therapy and helps patients to stick to their treatment. Ofloxacin is used to treat outer ear infections swimmers ear or ear canal infections and middle ear infectionsIt works by stopping the growth of bacteria.
This medication belongs to a class of. AJOGs Editors have active research programs and on occasion publish work in the Journal. Editorauthors are masked to the peer review process and editorial decision-making of their own work and are not able to access this work in the online manuscript submission system.
Its quite an experience hearing the sound of your voice carrying out to a over 100 first year.