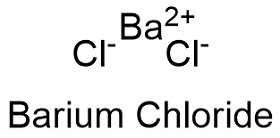
1130 K Boiling point. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems.
It is also hygroscopic converting first to the dihydrate BaCl 2 H 2 O 2.
Barium chloride dihydrate molar mass. Barium chloride is an inorganic compound with the formula Ba Cl 2. It is one of the most common water-soluble salts of barium. Like most other barium salts it is white toxic and imparts a yellow-green coloration to a flame.
It is also hygroscopic converting first to the dihydrate BaCl 2 H 2 O 2. It has limited use in the laboratory and. Barium stimulates striated cardiac and smooth muscle regardless of innervation.
It is antagonistic to all muscle depressants no matter whether they act primarily on nerve or muscle. Initial stimulation of contraction leads to vasoconstriction through direct action on arterial muscle peristalsis through action on smooth muscle tremors and cramps through action on the skeletal muscles and. Predict Barium Chloride Dihydrate Purified is an inorganic compound and one of the most common water soluble salts of barium.
Barium is mono-atomic and its ionic state is Ba2 B a X 2. An Email Id is required to process your orders. Like most other barium Barium Chloride CAS 10361-37-2 - GHS Pipe Marking Label Keep your hazardous materials labeled and facility safe with this durable.
BaBr 2 2H 2 O dihydrate Molar mass. 29714 gmol Appearance White solid Density. 478 gcm 3 anhydrous 358 gcm 3 dihydrate Melting point.
857 C 1575 F. 1130 K Boiling point. 1835 C 3335 F.
2108 K Solubility in water. 922 g100 mL 0C Magnetic susceptibility χ-92010 6 cm 3 mol Structure Crystal structure. Barium chloride is toxic.
Use care when handling. The used barium chloride should be put in the waste container provided. It is very important that the evaporating dish cools to room temperature before weighing.
If it is not cool convection currents will be set up that will lower the mass. The ring stand ring and crucible are hot. BaOH 2 8H 2 O.
BaIO 3 2 H 2 O. The formula for ironII chloride is FeCl2 which has a molar mass of 12675gmol. Moles of FeCl2mol 541mg FeCl 2 x 1g1000mg x 1mol 12675g FeCl2 0000427 mol FeCl2 Potassium nitrate is used for a variety of applications including fertilizer rocket fuel and fireworks.
Science ASSIST has an expert national advisory team with extensive collective experience across all school laboratory management and safety. It is this team that will help with your enquiry. What is the mass number for an atom of barium containing 56 protons and 81 neutrons.
What is the mass number for an atom of mercury containing 80 protons and 119 neutrons. What is the mass number for an atom of zinc containing 30 protons and 39 neutrons. An atom of the isotope ¹³³Cs contains how many protons p neutrons n and electrons e.
B 55 p 78 n 55 e. What mass of barium chloride is required to make a 100cm3 volume of 025molL-1 solution with respect to BaCl_2aq. When 100 mol of each compound below is dissolved in enough water to yield a 100 L solution which solution contains the greatest concentration of ions.
The solution in Figure 1211 contains 100 g of cobaltII chloride dihydrate CoCl 2 2H 2 O in enough ethanol to make exactly 500 mL of solution. What is the molar concentration of CoCl 2 2H 2 O. Mass of solute and volume of solution Asked for.
To find the number of moles of CoCl 2 2H 2 O divide the mass of the compound by its molar. Copper chloride appears as a yellowish-brown powder the anhydrous form or a green crystalline solid the dihydrate. Noncombustible but hydrogen chloride gas may form when heated in a fire.
Corrosive to aluminumUsed to manufacture other chemicals in dyeing in. Figure 78 illustrates the procedure for making a solution of cobaltII chloride dihydrate in ethanol. Note that the volume of the.
We can use the molar mass to convert the grams of CoCl 2 2H 2 O to moles. The molar mass of CoCl 2 2H 2 O is 16587 gmol and includes the two water molecules as they are part of the crystal lattice structure of this solid hydrate 2. Convert the volume.
The mole lab answer key. The molar mass of H_2O is 18 gmol The hydrogens make up 2g since each mole of hydrogen is 1g The oxygen makes up 16g. The percent composition of the compound is.
H 2g18g x 100 111 O 16g18g x 100 889. Percent composition can be calculated the chemical formula of a compound or it can be determined experimentally. Here is a video which discusses how to calculate percent.
Figure 78 illustrates the procedure for making a solution of cobaltII chloride dihydrate in ethanol. Note that the volume of the. We can use the molar mass to convert the grams of CoCl 2 2H 2 O to moles.
The molar mass of CoCl 2 2H 2 O is 16587 gmol and includes the two water molecules as they are part of the crystal lattice structure of this solid hydrate 2. Convert the volume. Academiaedu is a platform for academics to share research papers.
The rea chart in Lesser amount is the Limiting Here reactant 2 2 R reactant. A new solid substance formed from a solution. 00 g of Si and 1.
An example of the type of problem isAluminum sulfate and barium chlorid i Identify the limiting reactant. 37 g H2S 57. ANDOR Species E is not a catalyst because a catalyst occurs as.
Barium chloride dihydrate is a white crystalline solid with a bitter salty taste. Nutrient Agar Nutrient Broth. We Are The Manufacturer Importer Exporter and Wholesaler As Scientific Equipment Apparatus Laboratory Chemicals Brand.
HMBG Bendosen PC and other Laboratory Glasswares Plasticwares Porcelainware Educational Instruments Apparatus Charts Teaching Aids We. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems.
Ch3co2 name - restaurant-mandarinde. α D 20 between 38 and 42 in a 5 molar HCl solution. Not more than 8 100 C 5 hours in vacuum over P 2 O 5 for the dihydrate form.
Not more than 25. Not more than 10 calculated as PO 4 on the anhydrous basis Subsidiary colouring matters. Barium hydroxide calcium fluoride c TiO2.
Lithium carbonate ironIII hydroxide b NaOCl d AlOH3 d CoO b potassium permanganate c Ni3PO42 c S2Cl2 d H2Te. Review Questions 31 c dichlorine heptoxide. Protons 11 p 1 charge Electron 00 e 1 charge Neutron 01 n no charge.
Nearly all of the mass is located in the nucleus because this is the portion of the. The mass air flow meter also had a built-in intake air temperature sensor. The FA20D engine had a compression ratio of 1251.
The FA20D engine had a direct ignition system whereby an ignition coil with an integrated igniter was used for each cylinder. The spark plug caps which provided contact to the spark plugs were integrated with the ignition coil assembly. The FA20D engine had.