It reacts violently with ammonium nitrate and potassium chlorate causing fire and explosion hazards. Excellent temperature 72 o F 22 o C Benzol.
Because barium is difficult to purify many of its properties have not been accurately determined.
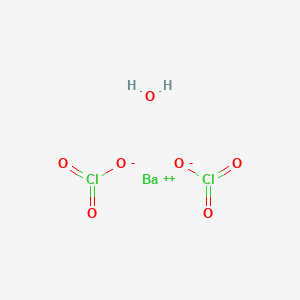
Barium chlorate weight. Barium is a soft silvery-white metal with a slight golden shade when ultrapure. 2 The silvery-white color of barium metal rapidly vanishes upon oxidation in air yielding a dark gray layer containing the oxideBarium has a medium specific weight and high electrical conductivity. Because barium is difficult to purify many of its properties have not been accurately determined.
Barium minerals are dense eg BaSO 4 45 grams per cubic centimetre. BaO 57 grams per cubic centimetre a property that was the source of many of their names and of the name of the element itself from the Greek barys heavyIronically metallic barium is comparatively light only 30 percent denser than aluminumIts cosmic abundance is estimated as 37 atoms on a scale where the. Barium sulfate or sulphate is the inorganic compound with the chemical formula Ba SO 4.
It is a white crystalline solid that is odorless and insoluble in water. It occurs as the mineral barite which is the main commercial source of barium and materials prepared from it. The white opaque appearance and its high density are exploited in its main applications.
A mixture of potassium chlorate and sodium amide explodes Mellor 8258. If a drop of a solution of sulfur dioxide in ether or alcohol is added to powdered potassium chlorate the mass explodes Mellor 2311. Potassium chlorate and sulfuric acid react to cause fire and possible explosions Mellor 2315.
Sodium chlorate appears as an odorless pale yellow to white crystalline solid. It is appreciably soluble in water and heavier so may be expected to sink and dissolve at a rapid rate. Although it is not itself flammable the solid product and even 30 solutions in water are powerful oxidizing agents.
Contact with wood organic matter ammonium salts sulfur sulfuric acid various metals and. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4.
Barium Nitrate BaOH2 Barium Hydroxide BaCl2 Barium Chloride BaCO3 Barium Carbonate BaI2 Barium Iodide BaO Barium Oxide BaO2 Barium Peroxide BaSO4 Barium Sulfate BBr3 Boron Tribromide BCl3 Boron Trichloride BeCl2 Beryllium Chloride BeI2 Beryllium Iodide BF3 Boron Trifluoride Br2 Bromine Gas C10H14N2 Nicotine C10H14O Carvone C10H15NO Ephedrine. Filler Coating Pigment Casein. Casein is a heterogeneous globular amphoteric phosphoprotein.
In Pulp Bleaching. In Pulp Bleaching and water treatment. Al 2 Si 2 O 5 OH 4.
CaMgCO 3 2. Chemical formula plays an important role in understanding different concepts of chemistry. Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand.
Its weight increase is less than 02 when it has been stored for 6 months at ambient temperatures. When the temperature is increased to 60C 140F the weight increase is less than 05 for a similar period. According to ASTM D570 its 24 hr water absorption rate is 003.
It resists most strong mineral acids and bases but like the other polyolens it is subject to attack by oxidizing. Barium has a 2 charge and hydroxide has a -1 charge therefore 1 Ba 2 ion is required to balance 2 OH- ions Ammonium has a 1 charge and phosphate has a -3 charge therefore 3 NH 4 ions are required to balance 1 PO 4 3- ion Potassium has a 1 charge and sulfate has a -2 charge therefore 2 K ions are required to balance 1 SO 4 2- ion. Oxidizing materials are liquids or solids that readily give off oxygen or other oxidizing substances such as bromine chlorine or fluorine.
They also include materials that react chemically to oxidize combustible burnable materials. This means that oxygen combines chemically with the other material in a way that increases the chance of a fire or explosion. Chlorate Solutions of chlorates yield no precipitate with silver nitrate TS.
The addition of sulfurous acid to this mixture produces a white precipitate that is insoluble in nitric acid but is soluble in 6 N ammonium hydroxide. Upon ignition chlorates yield chlorides recognizable by appropriate tests. When sulfuric acid is added to a dry chlorate decrepitation occurs and a greenish.
The last member of this class of compounds the perchlorate ion ClO 4- is made by electrolyzing solutions of the chlorate ion. The names of the oxyanions of the halogens use the endings - ite and - ate to indicate low and high oxidation numbers and the prefixes hypo - and per - to indicate the very lowest and very highest oxidation numbers as shown in the table below. Weed killer non-arsenical and not containing chlorate 200.
Weed killer liquid non poisonous 200. Weed killer liquid arsenical. Weed killer pow with chlt withnot les than 40 borax non-ars.
Weed killernon arsenicalnotcontaining chlorate. Barium Chlorate 1 0 2 OX Barium Nitrate 1 0 0 OX Barium Peroxide 1 0 0 OX Benzaldehyde 2 2 0 Benzoic Acid 2 1 - Benzol benzene 2 3 0. 35 to 52 by weight 2 0 1 OX Hydrogen Sulfide 3 4 0 Hydroquinone 2 1 0 Isoamyl Acetate 1 3 0 Isoamyl Alcohol 1 2 0 Isobutane 1 4 0 Isobutyl Acetate 1 3 0 Isobutyl Acrylate 1 3 1.
FP Standard NFPA 704 Placard Chemical Hazard Ratings 2373 Circadian. Excellent temperature 72 o F 22 o C Barium Sulfate. Fair temperature 72 o F 22 o C Barium Sulfide.
Good temperature 72 o F 22 o C Beer. Excellent temperature 72 o F 22 o C Benzol. Excellent temperature 72 o F 22 o C Borax.
Excellent temperature 72 o F 22 o C Boric acid. Excellent temperature 72 o F 22 o C Bromine. PHYSICAL AND CHEMICAL DATA 2-3 2-146 Linear Expansion of Miscellaneous Substances.
2-130 2-147 Cubical Expansion of Liquids. Human abundance by weight. 610000000 ppb by weight.
Oxygen atoms are present in water H 2 O and water is essential to all life. Oxygen and is present in many organic compounds. Most organisms use oxygen for respiration.
While oxygen O 2 is necessary for life oxygen as ozone O 3 is highly toxic. On the other hand ozone is an important. Acetyl coenzyme A lithium salt.
Empirical Formula Hill Notation. C 23 H 38 N 7 O 17 P 3 S xLi CAS No. 80957 free acid basis Compare Product No.
Al 3 aluminum ion. Transition B-group and Post-Transition Group IVA and VA Metals. These elements usually form ionic compounds.
Many of them can form more than one cation. The charges of the common transition metals must be memorized. Group IV and V metal cations tend to be either the group number or the group number minus two Many of these ions have common or.
Attempt Free Mock Test on General Awareness. Chemical Compounds List Their Common Names and Formulas Chemical Compound. A chemical compound is a chemical substance composed of many identical molecules composed of atoms from more than one element held together by.
In rubber components in engine systems designed for use in equipment that is not intended solely for consumer use and provided that no plasticised material comes into contact with human mucous membranes or into prolonged contact with human skin and the concentration value of bis2-ethylhexyl phthalate does not exceed. A 30 by weight of the rubber for i gasket coatings ii solid. It reacts violently with ammonium nitrate and potassium chlorate causing fire and explosion hazards.
500 M solution of calcium nitrate is combined with 200. A process during which two reactants form a precipitate ammonium nitrate sodium hydroxide. Add sodium hydroxide solution 1 in 10 dropwise with thorough mixing until the curdy precipitate that forms after the addition of each drop no.
Chlorate Ca2 calcium HCO 3 hydrogen carbonate ClO 4 perchlorate Sr2 strontium SO 3 2 sulfite IO 3 iodate Ba2 barium HSO 3 hydrogen sulfite MnO 4 permanganate Al3 aluminum SO 4 2 sulfate NO 2 nitrite Sn2 tinII HSO 4 hydrogen sulfate NO 3 nitrate Sn4 tinIV S 2O 3 2 thiosulfate OH hydroxide Pb2 leadII1 CrO 4 2 chromate IO 4 periodate Bi3.