
We might consider aspirin a true wonder drug as it has been shown to be useful in the treatment of a variety of conditions beyond fever and pain including prevention of coronary artery disease heart attack and stroke. Thisa websitea isa brillianta.
40 Aspirin took a- for the acetylation -spir- from Spirsäure and added -in as a typical drug name ending to make it easy to say.
Aspirin chemical information. Aspirin is a salicylate drug often used as an analgesic to relieve minor aches and pains as an anti-inflammatory compound that inhibits Cox-1Target. Cox-1Aspirin USAN also known as acetylsalicylic acid is a salicylate drug often used as ananalgesic to relieve minor aches and pains as an antipyretic to reduce fever and as an anti-inflammatory medication. The active ingredient of.
Aspirin is an orally administered non-steroidal antiinflammatory agent. Acetylsalicylic acid binds to and acetylates serine residues in cyclooxygenases resulting in decreased synthesis of prostaglandin platelet aggregation and inflammation. This agent exhibits analgesic antipyretic and anticoagulant properties.
Aspirin also known as acetylsalicylic acid ASA is a medication used to reduce pain fever or inflammation. Aspirin decomposes rapidly in solutions of ammonium acetate or the acetates carbonates citrates or hydroxides of the alkali metals. It is stable in dry air but gradually hydrolyses in contact with moisture to acetic and salicylic acids.
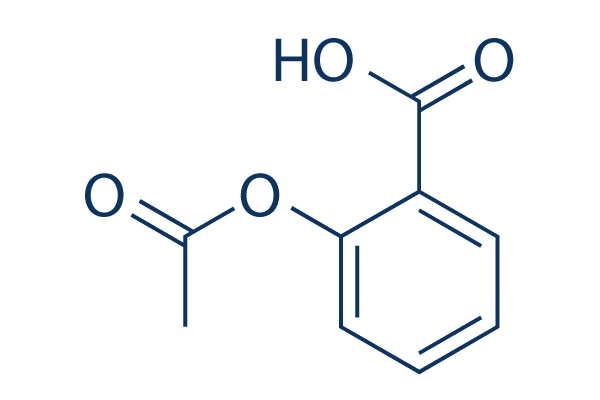
In solution with. The information of the atoms bonds connectivity and coordinates included in the chemical structure of aspirin can easily be identified by this visualization. By right-clicking the visualization screen various other options are available including the visualization of.
In the 1800s the Heyden Chemical Company was the first to mass-produce salicylic acid commercially. It was not until 1899 when a modified version named acetylsalicylic acid was registered and marketed by Bayer under the trade name aspirin. Even though it has been available since the early 1900s its real mode of action was not known until the late 1970s.
Some of the indications for aspirin. The name Aspirin was derived from the name of the chemical ASAAcetylspirsäure in GermanSpirsäure salicylic acid was named for the meadowsweet plant Spirea ulmaria from which it could be derived. 40 Aspirin took a- for the acetylation -spir- from Spirsäure and added -in as a typical drug name ending to make it easy to say.
In the final round of naming proposals that circulated. In 1828 Johann Andreas Buchner Professor of Pharmacology at the University of Munich purified salicin from willow bark. A number of scientists worked on refining the process but it was Professor Hermann Kolbe at Marburg University who first worked out the chemical structure of salicylic acid and made it synthetically in 1859.
The information below refers to products available in the United States that contain aspirin. Acetylsalicylic Acid Ecotrin Aspir 81 Bayer Aspirin Drug classes. Platelet aggregation inhibitors salicylates Aspirin systemic is used in the treatment of.
The information on the uses of aspirin is based on scientific studies that support treatment with aspirin for heart attacks strokes and some related conditions in patients who have. GI bleeding has been shown to occur in less than 1 41 of 6300 patients of those taking Aspirin to prevent a recurrent CVD event such as a heart attack4 A landmark meta-analysis showed that for patients taking low-dose Aspirin to prevent a first CV event there was a positive benefitrisk ratio for Aspirin greater than 215 As with all drugs the potentially life-saving benefits. My Library is designed to help you organize all your NSTA and personal learning resources.
Resources may be sorted and further subdivided into smaller personalized collections. Prostaglandins are chemical messengers the body produces which cause inflammation pain fever and swelling. Aspirin stops pain-producing prostaglandins being made by inhibiting the enzyme cyclooxygenase COX which is involved in their production.
Another important effect of aspirin is to help prevent blood clots from forming in arteries. The discovery of aspirin is customarily said to have resulted from Felix Hoffmanns rheumatic father encouraging his son to produce a medicine devoid of the unpleasant effects of sodium salicylate. Hoffmann a chemist in the pharmaceutical laboratory of the German dye manufacturer Friedrich Bayer Co in Elberfeld consulted the chemical literature and came across the synthesis of.
The German company Bayer patents aspirin on March 6 1899. Now the most common drug in household medicine cabinets acetylsalicylic acid was originally made from a. Does Dow Chemical make aspirin.
Mar 6 2008 202 am. Where aspirin is most absorbed between the stomach with pH 2 and small intestine with pH 6 if the pH of aspirin is 35. Jun 23 2008 909 am.
Thisa websitea isa brillianta. Jul 2 2010 808 am. I find that aspirin is best for my arthritic hip beause it seems.
The aspirin screen experiment has been designed to be a flexible open tool for teachers and students which allows students to run their own reaction online before taking part in the real thing. This also features an in-depth practical guide and a set of accompanying worksheets covering the theory. This resource allows students to run their own synthesis experiment on a computer or tablet.
Synthesis of aspirin from salicylic acid 4 Risk Assessment 5 1H-NMR Spectra. Students use this information combined with the principles of electronegativity to assign the chemical shifts in the molecule they have identified. Crude and recrystallized aspirin.
1H-NMR Spectra 12 11 10 9 8 7 6 5 4 3 2 1 0 f1 ppm Acetic Anhydride Salicylic Acid Aspirin Crude Aspirin Ac2O OAc OAc. Like all drugs aspirin can be toxic at high doses greater than 150 milligrams per kilogram body weight but the benefits of aspirin clearly outweigh the risks. We might consider aspirin a true wonder drug as it has been shown to be useful in the treatment of a variety of conditions beyond fever and pain including prevention of coronary artery disease heart attack and stroke.
7 Chemical ideas 135 Aim 2a. Purify the Aspirin Purifying my synthesised sample of Aspirin is essential as it is very likely to be impure. This could be due to solid objects from around the lab or filter paper from drying.
Also contamination of pipettes could lead to unwanted chemicals being incorporated into the sample. There may also. The Regulatory Information fields include information from the US.
Environmental Protection Agencys Title III Consolidated List of Lists the US. Department of Homeland Securitys Chemical Facility Anti-Terrorism Standards and the US. Occupational Safety and Health Administrations Process Safety Management of Highly Hazardous Chemicals Standard List see more about these data sources.
DMSO The aspirin of our era Share on Facebook Share on Twitter. Put simply DMSO is the real answer to treating pain inflammation and morenaturally. What if there was a simple solution to the daily misery of pain that was affordable easy to use and had no detrimental side effects.
Sounds like another quack-pot right. Trade names Aspirin. Registry numbers 7732-18-5.
Search Any Search Single-Component Structures Only Search Multi-Component Structures Only. Search Any Search Isotopically Labeled Structures Only Disregard Isotopically Labeled Structures. Aspirin S S Calcium hypochlorite bleach solution S S Calcium nitrate 50 S S Calcium sulfate S S.
S Satisfactory O Some attack U Unsatisfactory 2 of 5 HDPE Chemical Resistance Guide Reagent 70º F 21º C 140º F 60º C Reagent 70º F 21º C 140º F 60º C Camphor crystals S S Dextrose saturated S S Camphor oil U U Dibutyl ether O U Carbon dioxide 100 dry S S. Solutes such as aspirin dissolve in acid due to the chemical composition of the binder used in the tablet and the chemical nature of the solute. Aspirin or acetylsalicylic acid or 2-hydroxybenzoic acid 2 carboxyphenyl ester has a benzene ring C6H6 which is hydrophobic water-hating and this portion of the molecule does not interact with water.
However the molecule also has. Methyl salicylate is a benzoate ester that is the methyl ester of salicylic acidIt has a role as a flavouring agent a metabolite and an insect attractant. It is a benzoate ester and a member of salicylates.