In high doses aspirin can cause moderate to marked serum aminotransferase elevations occasionally with jaundice or signs of liver dysfunction and in lower doses in susceptible. A colorless bitter-tasting solid it is a precursor to and a metabolite of aspirin acetylsalicylic acid.

White thin flaky crystals Discussion My Results.
Aspirin acid mol. Aspirin also known as acetylsalicylic acid ASA is a medication used to reduce pain fever or inflammation. Specific inflammatory conditions which aspirin is used to treat include Kawasaki disease pericarditis and rheumatic fever. Aspirin given shortly after a heart attack decreases the risk of death.
Aspirin is also used long-term to help prevent further heart attacks ischaemic. Aspirin contains total 21 bonds. 13 non-H bonds 8 multiple bonds 3 rotatable bonds 2 double bonds 6 aromatic bonds 1 six-membered rings 1 carboxylic acids aromatic 1 esters aliphatic and 1 hydroxyl groups.
Learn more about aspirin chemical structure at Mol-Instincts. Salicylic acid is an organic compound with the formula HOC 6 H 4 CO 2 H. A colorless bitter-tasting solid it is a precursor to and a metabolite of aspirin acetylsalicylic acid.
It is a plant hormone. The name is from Latin salix for willow treeIt is an ingredient in some anti-acne productsSalts and esters of salicylic acid are known as salicylates. Aspirin is a salicylate drug often used as an analgesic to relieve minor aches and pains as an anti-inflammatory compound that inhibits Cox-1Target.
Cox-1Aspirin USAN also known as acetylsalicylic acid is a salicylate drug often used as ananalgesic to relieve minor aches and pains as an antipyretic to reduce fever and as an anti-inflammatory medication. The active ingredient of. Synthesis of AspirinAcetylsalicylic acid C 9 H 8 O 4 Step 1.
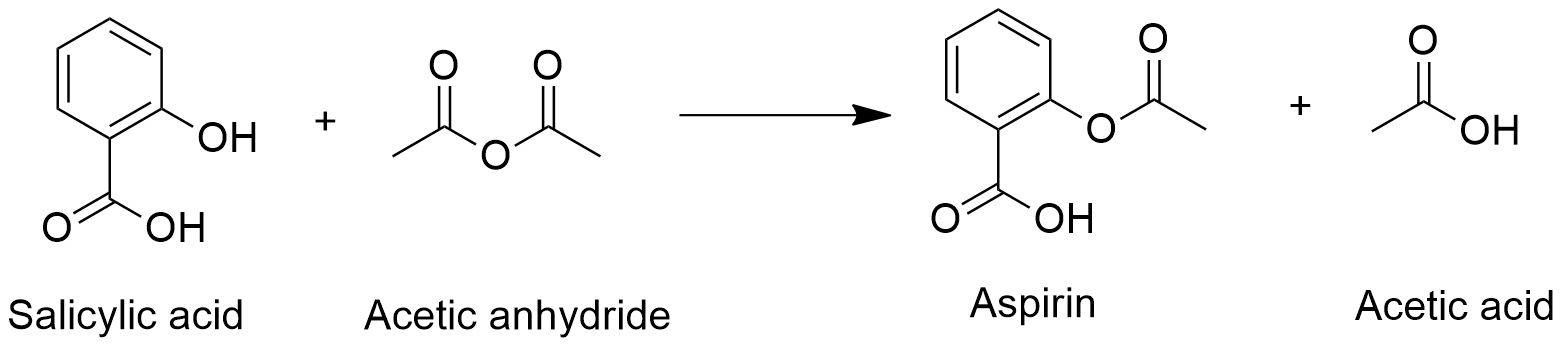
Dry an Erlenmeyer flask and add 3 grams of salicylic acid to it. Put 5 to 8 drops of 85 phosphoric acid along with 6 mL of acetic anhydride to the flask. Mix the solution and keep the flask in warm water for 15 minutes.
Hydrolysis of aspirin Aspirin 2-ethanoyloxybenzoic acid or acetylsalicylic acid hydrolyses to produce 2-hydroxybenzoic acid and ethanoic acid. Here is the equation for the reaction. O O The rate at which this reaction happens is important for two reasons.
When administered aspirin hydrolyses in the body. Also if aspirin tablets are stored in a humid environment such as a bathroom they. Aspirin is prepared by chemical synthesis from salicylic acid through acetylation with acetic anhydride.
The molecular weight of aspirin is 18016gmol. It is odourless colourless to white crystals or crystalline powder. Aspirin is an oral non-steroidal anti-inflammatory drug NSAID that is rapidly absorbed from the stomach and the small.
Grams salicylic acid 1000 tablets x 1 g aspirin1 tablet x 1 mol aspirin180 g of aspirin x 1 mol sal1 mol aspirin x 138 g of sal1 mol sal grams salicylic acid 76667 Answer. 76667 grams of salicylic acid are needed to produce 1000 1-gram aspirin tablets. Cite this Article Format.
Theoretical Yield Worked Problem. To determine the amount of aspirin acetylsalicylic acid in a sample the precise volume and concentration of the NaOH and the overall reaction must be known. The NaOH serves as a secondary standard because its concentration can change over time.
To find the precise concentration of the NaOH it must be titrated against a primary standard an acid that dissolves completely in water has a. Weigh 40 g 0030 mol of salicylic acid in a 125 mL Erlenmeyer flask. Using this quantity of salicylic acid to calculate the theoretical yield of aspirin.
Record the weigh on the report sheet. Carefully add 6 mL 0051 mol of acetic anhydride to the flask. Acetic anhydride is irritating to the skin and eyes 3.
Using extreme caution add 5 drops of concentrated sulfuric acid. 102 g mol d 108 gmL 138 gmol 180 gmol Aspirin Synthesis Experiment 5. 2 The spectroscopic analysis of aspirin will involve the complexing of ironIII to the deprotonated form of salicylic acid salicylate ion to give a purple solution.
Only the salicylate ion complexes to ironIII. Your aspirin product as well as a commercial aspirin tablet will be compared to a standard 015 ferric. 0017391 mol mol wt of salicylic acid 138 Expected number of moles of aspirin f 0010507 mol Expected mass of aspirin g 001739 x 180 31302 g mol wt 180 Percent yield d g Ã- 100 463 Melting Point.
Temperature range 1342 ÌŠC to 1361 ÌŠC Appearance. White thin flaky crystals Discussion My Results. According to my experiment and the results I.
Acetylsalicylic acid is hydrolyzed in the plasma to salicylic acid. Plasma concentrations of aspirin following after administration of the extended-release form are mostly undetectable 4-8 hours after ingestion of a single dose. Salicylic acid was measured at 24 hours following a single dose of extended-release acetylsalicylic acid 21.
Aspirin is an acid because of the carboxylic acid group which gives the molecule a relatively stable anion negatively charged form which means that it can readily donate a proton. The Bronstead-Lowry theory states that an acid is a proton donor while a base is a proton acceptor Therefore since aspirin donates a proton H it can be classed as an acid. Moles of acetylsalicylic acid mol _____ Concentration of acetylic acid in 1000 ml volumetric flask Stock solution _____ Mass of aspirin sample 1 0001 g _____ Mass of aspirin sample 2 0001 g _____ PartIIMeasureAbsorbanceofStandardsandAspirinSamples.
With the assistance of your instructor zero the instrument with the iron III chloride solution. Aspirin hay acetylsalicylic acid ASA acetosal là một dẫn xuất của acid salicylic thuộc nhóm thuốc chống viêm non-steroid. Có tác dụng giảm đau hạ sốt chống viêm.
Nó còn có tác dụng chống kết tập tiểu cầu khi dùng liều thấp kéo dài có thể phòng ngừa đau tim và hình thành cục nghẽn trong mạch máu. Molecular weight g mol-1 Quantity Amount mM Sodium salicylate 16010 241 g 15. 2M NaOH aq 4000 300 ml 60.
Ethanoic anhydride 10200 50 ml 53. Aspirin 18016 271 g 15. Amount of chemicals used.
Table 1 illustrates the different chemicals and the amounts of each used in the experiment. To prepare aspirin sodium salicylate was reacted with an excess of acetic anhydride at. Aspirin or acetylsalicylic acid is perhaps the most commonly used analgesic and antipyretic medication worldwide having been in clinical use for over 100 years.
Aspirin can cause several forms of liver injury. In high doses aspirin can cause moderate to marked serum aminotransferase elevations occasionally with jaundice or signs of liver dysfunction and in lower doses in susceptible. Aspirin is a prototype of non-steroidal anti-inflammatory drugs NSAIDs and member of the family of salicylates that have in common salicylic acid as the active agent.
Salicylic acid is composed of a benzene ring and two radicals one hydroxyl and one carboxyl. In the acetylsalicylic acid or aspirin the hydroxyl group salicylate is transformed into an acetyl group by esterification. The medical drug aspirin is made from salicylic acid.
1 mole of salicylic acid gives 1 mole of aspirin. Given that the chemical formula for salicylic acid is C 7 H 6 O 3 and the chemical formula for aspirin is C 9 H 8 O 4. In an experiment 1000 grams of salicylic acid gave 1212 grams of aspirin.
What was the percent yield. The appropriate acid chloride of aspirin 3ab 25 mmol in DMF 5 ml was added dropwise within 10 min to a stirring solution of the corresponding piperidone 4ai 25 mmol in DMF 15 ml containing triethylamine 3 mmol in an ice-cold water bath. The reaction mixture was stirred at the mentioned conditions for 4 h and stored at room temperature 2025 C overnight.
Salicylic Acid is a type of beta hydroxy acid BHA and phenolic acid with a chemical formula C 7 H 6 O 3. It is a BHA found as a natural compound in plants. It functions as a plant hormone.
This lipophilic monohydroxybenzoic acid is a derivative of salicin metabolism. It is a crystalline organic carboxylic acid with keratolytic bacteriostatic and fungicidal properties. It is poisonous when.
Eicosapentaenoic acid versus docosahexaenoic acid in mild-to-moderate depression. A randomized double-blind placebo-controlled trial. Nagakura T Matsuda S Shichijyo K Sugimoto H Hata K.
Dietary supplementation with fish oil rich in omega-3 polyunsaturated fatty acids in children with bronchial asthma. Aspirin ili acetilsalicilna kiselina Acidum acetylsalicylicum je lek iz porodice salicilata koji se često koristi protiv blažih bolova kao antipiretik protiv groznice i protiv zapaljenjaU malim dozama deluje kao antikoagulans sprečava zgrušavanje krvi pa se koristi u sekundarnoj prevenciji infarkta miokarda. Ime Aspirin je skovala nemačka kompanija Bajer.
Lauric acid is an inexpensive non-toxic and safe to handle compound often used in laboratory investigations of melting-point depression. Lauric acid is a solid at room temperature but melts easily in boiling water so liquid lauric acid can be treated with various solutes and used to determine their molecular masses.