Arsenic trioxide sold under the brand name Trisenox among others is an inorganic compound and medication. We value excellent academic writing and strive to provide outstanding essay writing service each and every time you place an order.
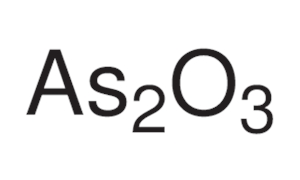
As an industrial chemical whose major uses include in the manufacture of wood preservatives pesticides and glass.
Arsenic trioxide solubility. Arsenic trioxide sold under the brand name Trisenox among others is an inorganic compound and medication. As an industrial chemical whose major uses include in the manufacture of wood preservatives pesticides and glass. As a medication it is used to treat a type of cancer known as acute promyelocytic leukemia.
For this use it is given by injection into a vein. Arsenic Trioxide - 1 - Section 1 - Chemical Product and Company Identification MSDS Name. Arsenic Trioxide Catalog Numbers.
Arsenic Oxide Arsenic Sesquioxide Arsenous Oxide Arsenous Acid Anhydride Arsenous Acid Company Identification. LabChem Inc 200 William Pitt Way Pittsburgh PA 15238 Company Phone Number. 412 826-5230 Emergency Phone Number.
Arsenic trioxide has been used in a variety of ways over the past 500 years. The reducing environment is also rich in organic matter which may enhance the solubility of arsenic compounds. As a result the adsorption of arsenic is reduced and dissolved arsenic accumulates in groundwater.
That is why the arsenic content is higher in reducing environments than in oxidizing environments. Arsenic is a naturally occurring element widely distributed in the earths crust. In the environment arsenic is combined with oxygen chlorine and sulfur to form inorganic arsenic compounds.
Arsenic in animals and plants combines with carbon and hydrogen to form organic arsenic compounds. Inorganic arsenic compounds are mainly used to preserve wood. Copper chromated arsenic CCA is used to.
The fatal human dose for ingested arsenic trioxide is 70 to 180 milligrams mg or about 600 micrograms per kilograms kgday ATSDR 2007. Onset of peripheral neuropathy may be delayed several weeks after the initial toxic insult. Mees lines may be visible in the fingernails several weeks to months after acute arsenic poisoning.
Mees lines are transverse white lines across the nails. Arsenic trichloride may be formed by the following reactions. 1 chlorine with arsenic trioxide 2 sulfur monochloride S2Cl2 or a mixture of S2Cl2 and chlorine with arsenic trioxide.
And 3 arsenic trioxide. With a mixture of sulfuric acid and a chloride. If the solubility of arsenic trioxide in water at 25 degrees C is 20 g 1 L water would 250 mL of water at 25 degrees C containing 35 g of arsenic trioxide be saturated unsaturated or supersat.
Recent clinical trials have found that arsenic trioxide has therapeutic value in the treatment of acute promyelocytic leukemia and there is interest in exploring its effectiveness in the treatment of a variety of other cancers 6970. In acute promyelocytic leukemia the specific molecular event critical to the formation of malignant cells is known. A study by Puccetti et al.
When Antimony or arsenic and solid potassium permanganate are ground together the metals ignite. Special Remarks on Explosion Hazards. Take care in handling as explosions may occur if it is brought in contact with organic or other readily oxidizable substances either in solution or in dry state.
Explosive in contact with sulfuric acid or hydrogen peroxide. Potassium permanganate acetic. We value excellent academic writing and strive to provide outstanding essay writing service each and every time you place an order.
We write essays research papers term papers course works reviews theses and more so our primary mission is to help you succeed academically.