Its slight alkalinity makes it useful in treating gastric or urinary hyperacidity and acidosis. These include paracetamol pKa 95 morphine pKa 99 and levothyroxine thy-roxine pKa 10.

The product is almost a mixture of common salt and vinegar if t.
Aqueous sodium bicarbonate. Intravenous sodium bicarbonate in an aqueous solution is sometimes used for cases of acidosis or when insufficient sodium or bicarbonate ions are in the blood. In cases of respiratory acidosis the infused bicarbonate ion drives the carbonic acidbicarbonate buffer of plasma to the left and thus raises the pH. For this reason sodium bicarbonate is used in medically supervised.
Sodium bicarbonate appears as odorless white crystalline powder or lumps. Slightly alkaline bitter taste. PH of freshly prepared 01 molar aqueous solution.
PH of saturated solution. SODIUM BICARBONATE reacts exothermically with acids to generate non-toxic carbon dioxide gas. Incompatible with acids acidic salts dopamine hydrochloride pentazocine lactate many alkaloidal salts aspirin and bismuth salicylate.
Sodium bicarbonate is the predominant buffer used in dialysis fluids and patients on maintenance dialysis are subjected to a load of sodium bicarbonate during the sessions suffering a transient metabolic alkalosis of variable severity. Side effects associated with sodium bicarbonate therapy include hypercapnia hypokalemia ionized hypocalcemia and QTc interval prolongation. Sodium tends to form water-soluble compounds such as halides sulfates nitrates.
Sodium chloride is extensively used for anti-icing and de-icing and as a preservative. Examples of the uses of sodium bicarbonate include baking as a raising agent and sodablasting. Along with potassium many important medicines have sodium added to improve their bioavailability.
Sample acidification by small volumes of 20 wt aqueous acetic acid abolishes the effects of exogenous and endogenous bicarbonate on nitrite measurement. Carbon dioxide CO2 and carbonates which are widely distributed in nature are constituents of inorganic and organic matter and are essential in vegetable and animal organisms. CO2 is the principal greenhouse gas in the.
Aqueous sodium bicarbonate is used in the extraction. This determines the amount of HCl needed. A saturated aqueous solution of sodium bicarbonate is 1 M 1 moleliter.
Concentrated HCl is 12 M. Because HCl reacts with sodium bicarbonate in a 11 molar ratio it follows that 1 mL of saturated aqueous sodium bicarbonate would be neutralized with 112 mL of concentrated HCl. If 115 mL of.
Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. Sodium bicarbonate also called sodium hydrogen carbonate or bicarbonate of soda NaHCO 3 is a source of carbon dioxide and so is used as an ingredient in baking powders in effervescent salts and beverages and as the main constituent of dry-chemical fire extinguishers.
Its slight alkalinity makes it useful in treating gastric or urinary hyperacidity and acidosis. It is also employed in. Sodium Bicarbonate Sodium Sulfate Calcium Chloride Separate Waste Streams for each of these Ethidium Bromide gels and contaminated filters solutions may go through filtration Osmuim-containing products Osium Tetroxide Osmium Dioxide Mercury-contaminated debris Formaldehyde Formalin or Paraformaldehyde aqueous solutions Chromium-containing solutions Lead-containing solutions.
Sodium hydroxide is a highly versatile substance used to make a variety of everyday products such as paper aluminum commercial drain and oven cleaners and soap and detergents. What is purpose of sodium hydroxide. Sodium hydroxide also known as caustic soda or lye is a highly versatile substance used in a variety of manufacturing processes to make other products like paper or aluminum.
Otherwise neutralize with alkali such as sodium carbonate or sodium bicarbonate soda ash lime or limestone granules. Dispose of spilled material and neutralization residues in accordance with applicable regulations. Protective clothing gloves and a combination acid gasP95 or P-100 respirator are recommended for persons responding to an accidental release see also.
Its common name derives from its chemical identity as a sodium hydrate and because it is caustic or corrosive. In pure form caustic soda is a waxy white solid. It readily absorbs water and forms aqueous solutions.
Commercially available caustic soda or sodium hydroxide is usually sodium hydroxide monohydrate NaOHH 2 O. Life is believed to have originated in the aqueous solutions of the worlds oceans and living organisms depend on aqueous solutions such as blood and digestive juices for biological processes. Water also exists on other planets and moons both within and beyond the solar system.
In small quantities water appears colourless but water actually has an intrinsic blue colour caused by slight. Aqueous humor is a clear fluid that fills and helps form the anterior and posterior chambers of the eye. The lens and cornea must remain clear to allow light transmission and therefore cannot be invested within a vasculature.
The aqueous humor is analogous to a blood surrogate for these avascular structures and provides nutrition removes excretory products from metabolism transports. Because of its high-level alkalinity sodium hydroxide in aqueous solution directly causes bond breakage in proteins especially disulfide bridges. Hair and fingernails are found to be dissolved after 20 hours of direct contact with sodium hydroxide at pH values higher than 92.
Sodium hydroxide has depilatory effects which have been described after accidental contact with solutions in the. 41 An aqueous solution containing n-alkylC12-C16benzyldimethylammonium chloride having average molecular weights ranging from 351 to 380 wherein the alkyl groups contain principally 12 to 16 carbons and not more than 1 percent each of the groups with 8 and 10 carbon atoms. Ammonium chloride CAS Reg.
Calcium stearate CAS Reg. Sodium nitrate is also synthesized industrially by neutralizing nitric acid with sodium carbonate or sodium bicarbonate. 2 HNO3 Na2CO3 2 NaNO3 H2O CO.
Or also by neutralizing it with sodium hydroxide however this reaction is very exothermic. What is the difference between sodium nitrate and potassium nitrate. A saturated solution of sodium bicarbonate a relatively weak base.
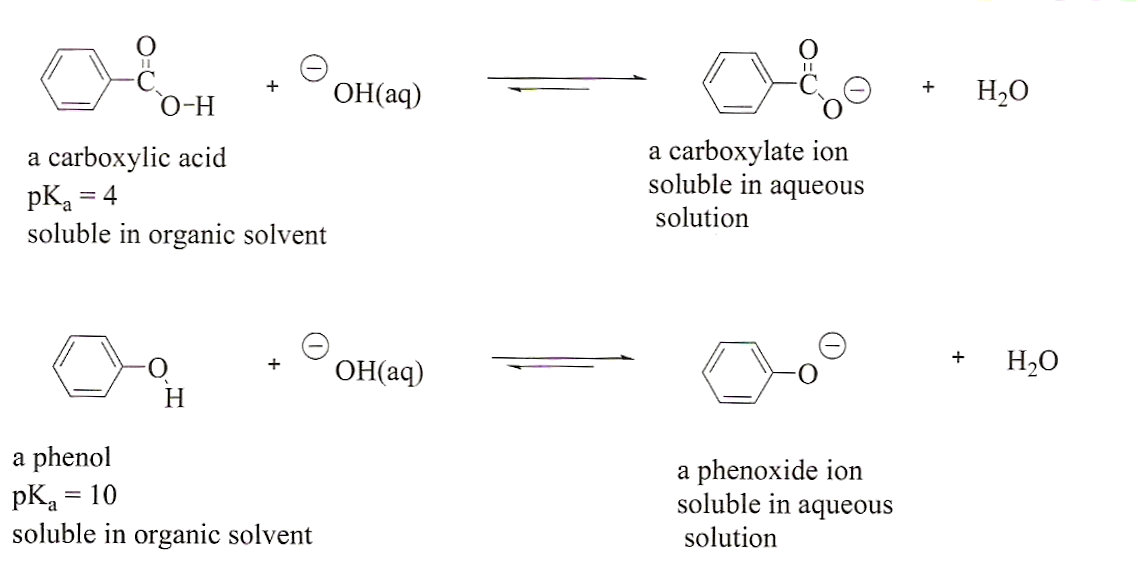
Phenols on the other hand require a stronger basic solution such as aqueous sodium hydroxide to be deprotonated. Hydrochloric acid is generally used to protonate amines. The solid forms of the acidic and basic organic compounds can be recovered from the aqueous solution using the same solubility switch principles.
React with sodium bicarbonate cf. Carboxylic acids and may be precipitated from solution of the phenoxide by saturation with carbon dioxide. A number of common drugs contain the phenol functional group.
These include paracetamol pKa 95 morphine pKa 99 and levothyroxine thy-roxine pKa 10. Since these phenolic drugs are 50 ionised. Sodium Bicarbonate baking soda abrasive blasting originated in 1984 during the restoration project of the Statue of Liberty with the assistance of Church Dwight.
Project engineers were searching for a replacement for sand blasting. Baking soda was the answer with its extraordinary cleaning and coating removal properties all while not causing any damage to Lady Libertys delicate copper. Sodium polystyrene sulfonate side effects.
Get emergency medical help if you have signs of an allergic reaction. Swelling of your face lips tongue or throat. Stop using sodium polystyrene sulfonate and call your doctor at once if you have.
Crystalline white powder or crystals. Soluble in water 1g15ml 25C insoluble in ethanol. Sodium citrate is the base of citric acid which is a weak organic acid with three carboxylic acid groups and as a result it dissociates three H and with three PKa values PKa1 314 PKa2 477 and PKa3 639.
Lactic acid or Sodium bicarbonate to adjust the pH. Since my hydrosol is already preserved and it makes up about 90 of the formulation I have reduced the concentration of the preservative accordingly. For this example Ive applied 02 of Euxyl K903 which has the following INCI name.
Benzyl alcohol benzoic acid dehydroacetic acid and tocopherol. You may decide to use. Answer 1 of 5.
Sodium acetate is a salt of acetic acid which is a weak acid. HCl being a strong acid will displace it from its salt forming sodium chloride and acetic acid. CH3COONa HCl CH3COOH NaCl.
The product is almost a mixture of common salt and vinegar if t.