
Calculate the percent composition of carbon for. The molweight of ethanol is 21201 6101 11600 4608 gmol.
Analysis of the products showed that 1139 g of phosphorus atoms were produced.
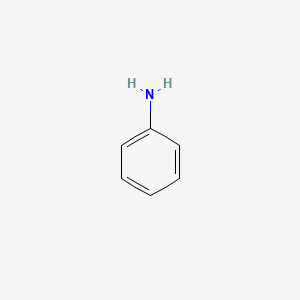
Aniline molar mass. Aniline is an organic compound with the formula C 6 H 5 NH 2Consisting of a phenyl group attached to an amino group aniline is the simplest aromatic amineIt is an industrially significant commodity chemical as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane dyes and other industrial chemicals. The molar mass of aniline is 93 gmol.
What is its molecular formula. C 6 H 7 N 13. Calculate the mass percent of carbon nitrogen and oxygen in acetamide C 2 H 5 NO.
C 40668 H 8533 N 23713 O 27086 14. A 5051 g sample of a compound made from phosphorus and chlorine is decomposed. Analysis of the products showed that 1139 g of phosphorus atoms were produced.
12311 gmol Appearance yellowish oily liquid. Pungent like paste shoe polish. It freezes to give greenish-yellow crystals.
It is produced on a large scale from benzene as a precursor to aniline. In the laboratory it is occasionally used as a solvent especially for electrophilic reagents. Nitrobenzene is prepared by nitration of.
SOLUTION 10 IN CCl4 FOR 3800-1300 10 IN CS2 FOR 1300-650 10 IN CCl4 FOR 650-250 CM-1 VERSUS SOLVENT. PERKIN-ELMER 521 GRATING Instrument parameters. FILTERS AT 3150 2500 2000 1150 700 410.
GRATING CHANGES AT 2000 630 CM-1. 0012 0011 AND 0010 CM CsBr CELL Resolution. Aniline is a clear to slightly yellow liquid with a characteristic odor.
It does not readily evaporate at room temperature. Aniline is slightly soluble in water and mixes readily with most organic solvents. Aniline is used to make a wide variety of products such as polyurethane foam agricultural chemicals synthetic dyes antioxidants stabilizers for the rubber industry herbicides.
Molar mass and percent composition worksheet. Determine the molecular formula for a compound with the empirical formula NPCl 2 and a molar mass of 347. Molar Mass and Percentage Composition Calculate the molar masses and percentage composition of each of the following compounds.
Calculate the percent composition of carbon for. The atomic mass of hydrogen is 100794 amu and that of oxygen is 159994. Since water molecules contain 2 hydrogen atoms and only one oxygen atom the molecular mass of H 2 O is 180154 amu.
The molar mass of a substance is defined as the total mass of one mole of the substance. It is often represented in terms of grams per mole. Couplingcomplexing with 2-amino-4-nitrophenol and 24-dimethoxy aniline.
The optical reduced scattering coefficient and mass density distribution factor analysis. These markers were combined utilizing logistic regression and the combined rectal LEBS marker had a sensitivity of 88 and specificity of 72 for advanced adenomas. The rectal LEBS was not confounded by location of the adenomas.
Chemical Elements Periodic Table Compound Name Formula Search Moles to Grams Calculator Common Compounds List Chemical Equation Balancer Complete List of Acids Complete List of Bases Molar to Mass Concentration Converter Molar Mass Calculator Cations Anions List Dilution. A simplified version of the isolated vascularly perfused rat small intestine was developed. With 13-dinitrobenzene 13-DNB as a model xenobiotic.
Both 3-nitroaniline 3-NA and 3-nitroacetanilide 3-NAA were formed and absorbed following intralumenal doses of 13-DNB 18 or 42 umol to isolated vascularly perfused rat small intestine. Dose fasting or antibiotic pretreatment had no. 11 g of aniline monomer with 01 g of SDS and 01 g of CoFe 2 O 4 nanoparticles were dissolved in 10 ml of distilled water and the solution was stirred for 30 min on magnetic stirrer to foam up strongly.
The resulting solution was then placed in an ice bath until the temperature was reached to 04 C. Also 228 g of ammonium persulfate APS with a molar mass of 22818 g. Must show ratio of massmolar mass for both elements in calculations PV nRT.
P nRT V nN 2 nO 2RT V P 0875 0875 mol 00821 Latm molK 298 K 500 L 856 atm 1 point for correct pressure setup and value Note. Only 1 point earned if calculation of moles is incorrect but then wrong values used correctly in P nRTV. We would like to show you a description here but the site wont allow us.
An empirical equation was developed which gives net heat of combustion based on the determined values of aniline point ASTM D 611 and API gravity ASTM D 287. If the fuel contains sulfur a correction is applied for sulfur determined according to ASTM D129 D 1266 D 2622 or D 3120 the method selected depends on the volatility of the sample. This can be converted to kJ per mass units.
The molweight of ethanol is 21201 6101 11600 4608 gmol. The heat of combustion of ethanol ΔH c C 2 H 6 O l 136691kJmol 1000gkg 4808 gmol 29664 kJkg ethanol 297 MJkg 12754 BTUlb 7086 kcalkg. The table below shows values of heat of combustion calculated after the above described method.
Electric Heating of Mass - Electric heating of an object or mass - temperature change vs. Food and Foodstuff - Specific Heat - Specific heat of common food and foodstuff like apples bass beef pork and many more. Heat Capacity - Heat capacity is the amount of heat required to change temperature of a substance by one degree.
Get 247 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help proofreading and editing your draft fixing the grammar spelling or formatting of your paper easily and cheaply.