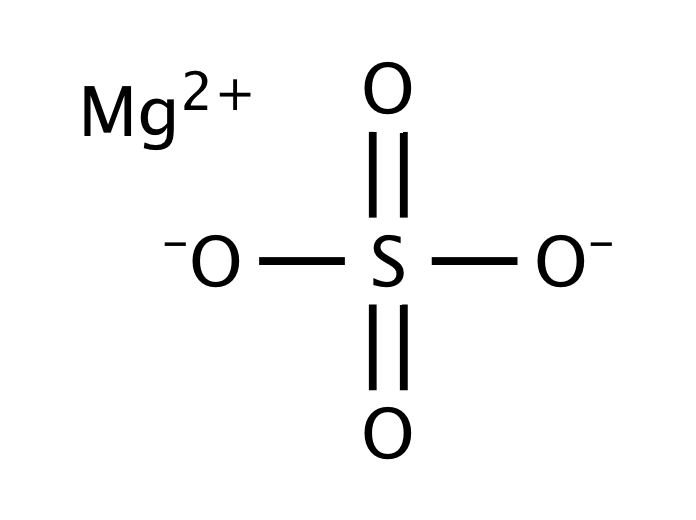
Here is a video which discusses how to calculate percent. Magnesium sulfate epsom salts can be a hydrated salt.

Aluminium sulfate is also called Filter Alum or Dialuminum trisulfate.
Anhydrous magnesium sulfate molar mass. Magnesium sulfate or magnesium sulphate in British English is a chemical compound a salt with the formula MgSO 4 consisting of magnesium cations Mg 2 2019 by mass and sulfate anions SO 2 4It is a white crystalline solid soluble in water but not in ethanol. Magnesium sulfate is usually encountered in the form of a hydrate MgSO 4 nH 2 O for various values of n between 1 and 11. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass.
These salts are typical ionic halides being highly soluble in waterThe hydrated magnesium chloride can be extracted from brine or sea waterIn North America magnesium chloride is produced primarily from Great. Molecular Weight Molar Mass. It is important to note that anhydrous magnesium sulfate undergoes decomposition at temperatures above 1124 degrees Celsius.
Since MgSO 4 is an ionic salt it exhibits high solubility in water. It can also be noted that the solubility of. O and magnesium sulfate MgSO.
O will be studied. The water molecules are loosely attached to the salt and can be removed upon heating yielding the anhydrous salt salt without water. The mass of water lost during heating can be determined and the coefficient.
Can be calculated from the ratio of moles of anhydrous salt to. Magnesium sulfate epsom salts can be a hydrated salt. Upon heating the water is separated from the salt leaving only anhydrous magnesium sulfate.
By comparing the mass of the hydrate and the anhydrous form the number of moles of water combined with one mole of. If 290 g of MgSO 4 7H 2 O is thoroughly heated what mass of anhydrous magnesium sulfate will remain. Solution using 11 molar ratio.
1 Convert the 290 g of magnesium sulfate heptahydrate to moles. 290 g 2464696 gmol 011766157 mol. 2 Note that after heating you end up with anhydrous magnesium sulfate MgSO 4 and you will have remaining the same number of moles.
Other examples of hydrates are. Lithium perchlorate trihydrate - LiClO 4 3H 2 O. Magnesium carbonate pentahydrate - MgCO 3 5H 2 O.
And copperII sulfate pentahydrate - CuSO 4 5 H 2 O. The water in the hydrate referred to as water of hydration can be removed by heating the hydrate. When all hydrating water is removed the material is said to be anhydrous and is referred to as.
Thermal decomposition reactions and also for mass gain when reacting magnesium in oxygen. 351 g of hydrated zinc sulfate were heated and 197 g of anhydrous zinc sulfate were obtained. Calculate the value of the integer xin ZnSO4xH2O Calculate the mass of H2O 351 197 154g Calculate moles of ZnSO4 Calculate moles of H2O 197 1615 154 18 00122.
When a 322 g sample of an unknown hydrate of sodium sulfate Na2SO4 H2Os is heated H2O molar mass 18 g is driven off. The mass of the anhydrous Na2SO4s molar mass 142 g that remains is 142 g. The value of x in the hydrate is.
A 0013 B 18 C 60 D 10. A sample of carbonate rock is a mixture of CaCO3 and MgCO3. The rock is analyzed in a laboratory and the.
ZnS04 7 1420 zinc sulfate heptahydrate A sample of the hydrate of NazC03 has a mass of 8. The bright-line spectra observed in a spectroscope for three elements and a mixture of two of these elements are represented in the diagram below. When expressing the formula for a hydrate it is necessary to notate the fixed number of H2O molecules.
Students do NOT have access to these. Iron metal is. What is Aluminium Sulfate Al 2 SO 4 3.
Al 2 SO 4 3 is a chemical compound with chemical name Aluminium sulfate. Aluminium sulfate is also called Filter Alum or Dialuminum trisulfate. It is a white crystalline solid in its anhydrous form and in its solution form it appears as a colourless liquid.
Both the forms are non-toxic and non-combustible. Aluminum potassium sulfate AlKSO42 or KAlSO42 or AlKO8S2 CID 24856 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Magnesium carbonate is a magnesium salt with formula CMgO3.
Its hydrated forms particularly the di- tri- and tetrahydrates occur as minerals. It has a role as an antacid and a fertilizer. It is a magnesium salt a carbonate salt and a one-carbon compound.
Take the mass of the hydrate and subtract the mass of the anhydrate to get the mass of water. 1770 g Na 2 CO 3 nH 2 O - 1510 g Na 2 CO 3 260 g H 2 O. If 8 moles of magnesium chloride react with enough aluminum how many moles of aluminum chloride are produced.
3 MgCl2 2 Al 3 Mg 2 AlCl3. N2 3 H2 — 2 NH3 write the following molar ratios. A N2 H2 b N2 NH3 and c H2 NH3.
Why do we know that in the balanced chemical equation C O2 — CO2 1 g of C will not react exactly with 1 g of O2. Esters of the low molar mass alkanoic acids and alkanols have fragrant. Add a small amount of anhydrous magnesium sulfate to a clean dry conical flask.
Run the remaining organic contents of the separating funnel into the conical flask. Stopper the conical flask and allow to stand for 15 minutes. Decant the contents of the conical flask into a clean dry 50 mL round bottom flask.
The molar mass of H_2O is 18 gmol The hydrogens make up 2g since each mole of hydrogen is 1g The oxygen makes up 16g. The percent composition of the compound is. H 2g18g x 100 111 O 16g18g x 100 889.
Percent composition can be calculated the chemical formula of a compound or it can be determined experimentally. Here is a video which discusses how to calculate percent. Magnesium sulfate reacts with barium chloride according to the following balanced equation.
If 120 g of magnesium sulfate is allowed to react with 100 g of barium chloride in a water solution wh. Conversion versus experimental number-average molar mass M n exp and M w M n of the RAFT polymerization of PMAA with CTA3 and a targeted DP of MAACTA 120 and a molar ratio of CTAACVA 10 at 70 C ab. For the polymerizations at 80 C a kinetical study with a molar ratio of CTAACVA 10 cd and 5 ef is presented.
The molar mass of CoCl 2 2H 2 O is 16587 gmol and includes the two water molecules as they are part of the crystal lattice structure of this solid hydrate 2. Convert the volume into Liters 3. Plug values into the Molarity equation.
772 Parts Per Solutions. In the consumer and industrial world the most common method of expressing the concentration is based on the quantity of solute in. Lauryl pyridinium 5-chloro-2-mercaptobenzothiazole.
Lignin calcium sulfonate. Lignin sodium sulfonate. Linoleamide linoleic acid amide Magnesium fluoride.
For use only as bonding agent for aluminum foil stabilizer or preservative. Total fluoride from all sources not to exceed 1 percent by weight of the.