
Now the difference between hydrated copperII sulfate and anhydrous copperII sulfate except for the fact that the former contains water of crystallization is the color. Product and Company Identification Copper II Sulfate Anhydrous SynonymsGeneral Names.
Uses of Copper Sulfate.
Anhydrous copper sulfate. Anhydrous copper sulfate is 3981 percent copper and 6019 percent sulfate by mass and in its blue hydrous form it is 2547 copper 3847 sulfate 1282 sulfur and 3606 water by mass. Four types of crystal size are provided based on its usage. Large crystals 1040 mm small crystals 210 mm snow crystals less than 2 mm and windswept powder less than 015 mm.
Copper II Sulfate Anhydrous MSDS 22700 Section 1. Product and Company Identification Copper II Sulfate Anhydrous SynonymsGeneral Names. Cupric Sulfate Product Use.
For educational use only Manufacturer. Columbus Chemical Industries Inc Columbus WI 53925. 24 Hour Emergency Information Telephone Numbers CHEMTREC USA.
Anhydrous Cobalt chloride is blue colored which on absorbing moisture gets turned into deep purple colored. So when you add a drop of water to Cobalt chloride its color changes due to hydrate formation. In this manner does copper sulfate react with water.
When copper sulfate CuSO4 reacts with water H2O the product is still copper sulfate. CID 1118 Sulfuric acid CID 23978 Copper Dates. Copper is an essential trace element that is included in some over-the-counter multivitamin and mineral supplements even though copper deficiency is quite rare and supplementation is rarely needed.
Copper sulfate both anhydrous and pentahydrate and copper dusts and mists are considered as copper. The Maximale Arbeitsplatz-Konzentration or maximum workplace concentration MAK for copper sulfate has been set at 01 mgm 3 inhalable fractions. Maximum Contaminant Level MCL.
The MCL is the highest level of contaminant that is legally allowed in drinking water. The MCL is. Copper sulfate is highly soluble in water with solubility values of 1055 molal and 1502 molal ate 10 o C and 30 o C respectively.
A typical example of a single displacement reaction where one metal displaces another is the reaction between iron and copper sulfate given by the reaction Fe CuSO 4 FeSO 4 Cu. Uses of Copper Sulfate. In the case of copperII sulfate hydrates you only get x as a whole number.
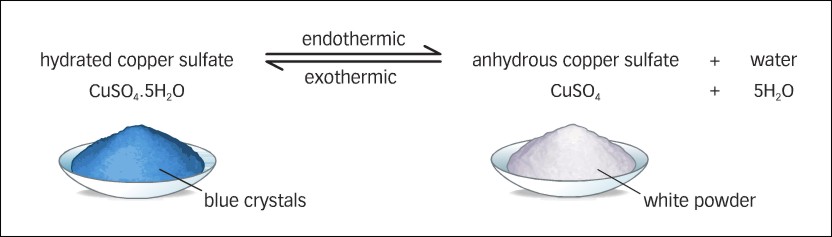
Now the difference between hydrated copperII sulfate and anhydrous copperII sulfate except for the fact that the former contains water of crystallization is the color. For example copperII pentahydrate CuSO_4 5H_2O is blue. On the other hand.
The colour change on adding water to anhydrous copperII sulfate has been used as a test for the presence of water in a liquid. The more observant should notice that the addition of water to anhydrous copperII sulfate is exothermic as the tube becomes noticeably hot if the water is added very slowly. They should therefore conclude that the same quantity of energy is absorbed when the.
Anhydrous magnesium sulfate is commonly used as a desiccant in organic synthesis owing to its affinity for water and compatibility with most organic compounds. During work-up an organic phase is treated with anhydrous magnesium sulfate. The hydrated solid is then removed by filtration decantation or by distillation if the boiling point is low enough.
Other inorganic sulfate salts such as. Heating copperII sulfate pentahydrate CuSO 4 5H 2 O yields anhydrous copperII sulfate CuSO 4. How Anhydrous Chemicals Are Prepared.
The method of preparation depends on the chemical. In some cases simply applying heat can drive off water. Storage in a desiccator can slow rehydration.
Solvents may be boiled in the presence of a hygroscopic material to prevent water from. Pure copperII sulfate is white. It is also known as anhydrous copperII sulfate because it has no water in it.
When water is present in a sample of copperII sulfate it turns blue. It is still. For instance the copper sulfate used earlier in the semester was stated to be CuSO 4 but it actually had absorbed 5 moles of water for every 1 mole of CuSO 4 and should have been correctly labeled as CuSO 45H 2O copper II sulfate pentahydrate.
The water in these compounds can be removed quantitatively by heating the compound with a bunsen burner. The loss in mass of the compound after. Anhydrous copper sulfate causes hydroxylamine to ignite the hydrated salt is vigorously reduced.
Copper sulfate Fire Protection Guide to Hazardous Materials. National Fire Protection Association 1997 p. Hazardous Substances Data Bank HSDB Solutions of sodium hypobromite are decomposed by powerful catalytic action of cupric ions even as impurities.
The blue colour of the hydrated compound should gradually fade to the greyish-white of anhydrous copperII sulfate. Avoid over-heating which may cause further decomposition and stop heating immediately if the colour starts to blacken. If over-heated toxic or corrosive fumes may be evolved.
A total heating time of about 10 minutes should be enough. Allow the crucible and contents. Chem One is the leading supplier of Copper Sulfate for the US market with our extensive lines of Chem One branded products.
We are also the exclusive or select distributor for key manufacturers located throughout the world. We store and offer these products at our facilities in Houston Texas Laredo Texas and Tampa Florida. Product Applications for Copper Cupric Sulfate Pentahydrate.
This material contains Cupric sulfate anhydrous listed as Copper compounds nos - CAS 7758-98-7 which is subject to the reporting requirements of Section 313 of SARA Title III and 40 CFR Part 373. This material does not contain any hazardous air pollutants. This material does not contain any Class 1 Ozone depletors.
Answer 1 of 17. Explanation - Zinc is more reactive than copper. So here on adding zinc to CuSO4 solution zinc displaces copper from copper sulphate forms zinc sulphate solution.
This is indicated by colour change from blue to colourless. CuSO4 solution has a blue colour while ZnSO4 solu. Copper sulfate also known as blue vitriol Salzburg vitriol Roman vitriol blue copperas or bluestone is a chemical compound comprised of Copper Sulphur and Oxygen whose formula is CuSO4.
Copper Sulphate is an odorless crystalline substance electric blue in color highly toxic and not safe to work with. It is produced industrially by treating copper metal with hot concentrated sulfuric. Synonyms Cupric sulfate anhydrous.
Copper monosulfate Recommended Use Laboratory chemicals. Uses advised against Food drug pesticide or biocidal product use. Details of the supplier of the safety data sheet Emergency Telephone Number For information US call.
001-800-ACROS-01 Europe call. 32 14 57 52 11 Emergency Number US001-201-796-7100 Europe. Magnesium Chips - Large.
Magnesium Chips - Small. Welcome to Alpha Chemicals. We are here to supply you quality chemicals at a reasonable price.
Zinc Sulfate Monohydrate Our Associate Companies A progressive and dynamic company Buradon is one of India s leading manufacture and exporter of fine chemicals to the rigid standard of GR AR EL ACS and LR grades due to its industrial exposure commitment and market fixing capabilities from India. CopperII chloride is light brown when anhydrousIt is green when hydratedIt is a weak oxidizing agentIt reacts with aluminium foil to make hydrogen copperI oxide and aluminium chlorideThis is used in school demonstrations. It releases chlorine and turns into copperI chloride when heated very hot.
It reacts with sodium hydroxide to make copperII hydroxide. Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses.
IP BP EP Ph Eur USP NF JP Analytical Reagent FCC Food Grade Chemicals Excipients Shale Gas Fracturing Chemicals Manufacturers. Muby Chemicals of Mubychem Group established in 1976 is the original manufacturers of Specialty Chemicals Pharmaceutical Excipient Fragrance Food Flavor chemicals Reagent Grade Chemicals Shale Gas Fracturing Chemicals in India. For example copper sulfate is a vernacular name which may refer to copperI sulfate or copperII sulfate.
An archaic name is an older name for a chemical that predates the modern naming conventions. Its helpful to know archaic names of chemicals because older texts may refer to chemicals by these names. Some chemicals are sold under archaic names or may be found in.