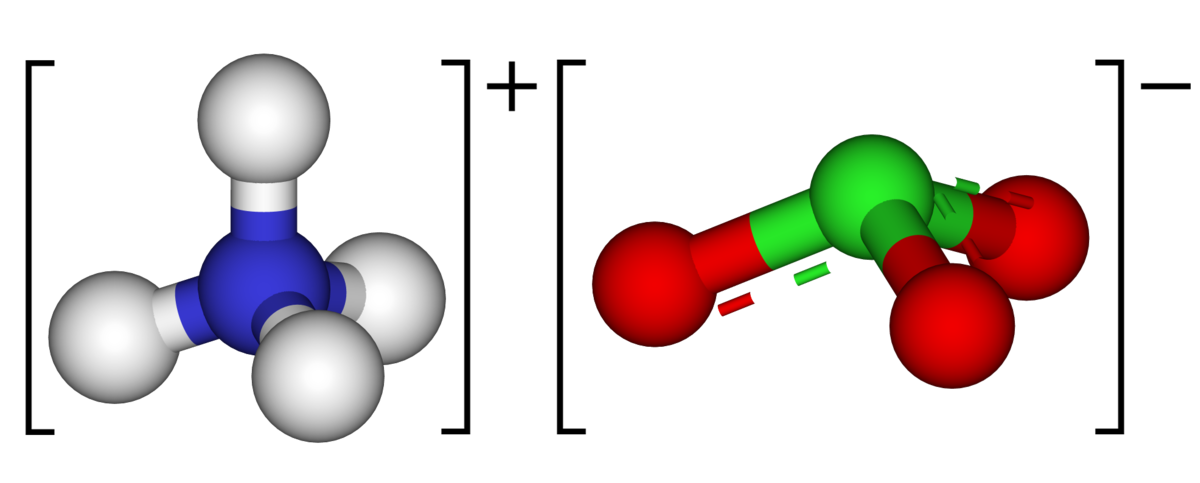
Ammonium perchlorate an oxidizer is the most prevalent form of this compound. 4 Perchlorate ion.

It is a powerful oxidizer.
Ammonium perchlorate organic or inorganic. Ammonium perchlorate AP is an inorganic compound with the formula NH 4 ClO 4. It is a colorless or white solid that is soluble in water. It is a powerful oxidizer.
Combined with a fuel it can be used as a rocket propellant. Its instability has involved it in a number of accidents such as the PEPCON disaster. Ammonium perchlorate AP is produced by reaction between ammonia.
Ammonium cation is found in a variety of salts such as ammonium carbonate ammonium chloride and ammonium nitrateMost simple ammonium salts are very soluble in water. An exception is ammonium hexachloroplatinate the formation of which was once used as a test for ammoniumThe ammonium salts of nitrate and especially perchlorate are highly explosive in these cases ammonium is the reducing. Perchlorate is both a naturally occurring and manmade anion that is typically found in the form of perchloric acid and salts such as ammonium perchlorate potassium perchlorate and sodium perchlorate.
Ammonium perchlorate an oxidizer is the most prevalent form of this compound. Has been widely used in solid propellants fireworks and flares. And is a constituent of many munition.
Ammonium nitrate chromic acid hydrogen peroxide nitric. Also avoid friction and store cold. Phosphorus white Air oxygen.
Phosphorus pentoxide Alcohols strong bases water. Potassium Air moisture andor oxygen or water carbon tetrachloride carbon dioxide. Potassium Chlorate Sulfuric and other acids.
Development of organicinorganic nanocomposites often achieved by grafting synthetic polymers on inorganic particles or by adding modified nanoparticles NPs into polymer matrices is intended to produce composite materials with improved mechanical and other properties. Nanocomposites made up of inorganic nanoparticles and organic polymers represent a new class of. Nitric acid and organic or inorganic waste components.
Immediately dilute any mixtures generated from concentrated acids by slow addition to ice or water in an open plastic container or a plastic bottle behind a shield or hood sash. Chemical Compatibility Chart 1 Inorganic Acids 1 2 Organic acids X 2 3 Caustics X X 3 4 Amines Alkanolamines X X 4 5 Halogenated Compounds X X X 5 6 Alcohols. The distinction between the organic and inorganic are not absolute and there is much overlap especially in the organometallic chemistry which has applications in every aspect of the pharmacy chemical industryincluding catalysis in drug synthesis pigments surfactants and agriculture.
In short Inorganic chemistry is the branch of chemistry that deals with inorganic compounds. Peroxides organic Acids organic or mineral avoid friction store cold Phosphorus white Air oxygen alkalis reducing agents Potassium Carbon tetrachloride carbon dioxide water Potassium chlorate Sulfuric and other acids ammonium salts metal powders sulfur finely divided organics combustibles Potassium perchlorate see also chlorates. Perchlorate OH.
The inorganic compound ammonium cyanate is now known to be an isomer of urea. Both contain the same type and number of atoms but in different structural arrangements Encouraged by Wöhlers discovery others succeeded in making simple organic compounds from inorganic ones and by roughly 1860 it was generally recognized that a vital force was unnecessary for the. Inorganic compounds are compounds that do not deal with the formation of carbohydrates or simply all other compounds that do not fit into the description of an organic compound.
For example organic compounds include molecules with carbon rings andor chains with hydrogen atoms see picture below. Inorganic compounds the topic of this section are every other molecule that does not include. Ammonium perchlorate can decompose at high temperatures forming toxic gases such as chlorine hydrogen chloride and nitrogen oxides.
Closed containers or tanks may rupture and explode if heated. It does not burn but is a powerful oxidizer and explosive when mixed with combustible materials. It is highly reactive and impact or high temperatures can cause violent decomposition or explosion.
Acids organic or mineral avoid friction store cold. Phosphorus white Air oxygen alkalis reducing agents. Carbon tetrachloride carbon dioxide water.
Sulfuric and other acids. Potassium perchlorate see also chlorates Sulfuric and. We will limit our attention here to inorganic compounds compounds that are composed principally of elements other than carbon and will follow the nomenclature guidelines proposed by IUPAC.
The rules for organic compounds in which carbon is the principle element will be treated in the module on organic chemistry. To name an inorganic compound we need to consider the. Chemical Storage Guidelines from The CDC Chemical Storage Guidelines from The CDC Guidelines for Safe Chemical Storage.
If you need a set of chemical storage guidelines meet OSHA and safety needs in your lab school manufacturing or storage facility this page should provide the template you need. Ammonium perchlorate AP-based composite propellants have been a workhorse in the field of solid rocket propulsion for more than five decades. This type of propellant typically contains a multi-modal distribution of AP NH 4 ClO 4 grains 20 to 200 mm embedded in the hydroxyl-terminated polybutadiene HTPB matrix.
The physiochemical processes that occur during the combustion of. 4 Perchlorate ion. Ammonium Sulfate Ammonium ions and Sulfate ions NH 4.
45H 2O 11 Undeca. Common Covalent Binary Inorganic Compounds of atoms Prefix element closest to fluorine goes on rightCommon Examples 1 Mono H 2 Hydrogen N 2 Nitrogen 2 Di O 2 Oxygen NH 3 Ammonia 3 Tri O 3 Ozone NO Nitrogen monoxide Nitric Oxide 4 Tetra H 2O Water Dihydrogen Monoxide NO 2. Metal hydroxides such as Sodium Potassium Calcium Nickel hydroxide Ammonium hydroxide Organic bases.
Amines such as Ethanolamine Tributylamine etc. Glacial acetic acid 100 Acetic acid 80 Acetic anhydride Formic acid 85 Propanoic acid 100 also called Propionic acid. Ammonium nitrate with more than 02 percent combustible substances including any organic substance calculated as carbon to the exclusion of any other added substance 11D UN0222.
The SEMS Search allows you to retrieve Superfund data from the Superfund Enterprise Management System SEMS database in Envirofacts. Specify a facility by using any combination of facility name and geographic location. Understanding and controlling defect generation and movement in organic-inorganic lead halide perovskite materials will not only lead to more stable devices but also new device concepts.
In article number 1700527 Wei D. Lu and co-workers reveal the formation migration and annihilation of iodine vacancies in CH 3 NH 3 PbI 3 films under an electric field andor light illumination. As appropriate use either organic or inorganic convention to list the elements.
Then add appropriate subscripts to indicate the number of atoms of each element present in the molecular formula. The molecular formula lists the elements in the molecule and the number of atoms of each. A Each molecule of sulfur monochloride has two sulfur atoms and two chlorine atoms.
Inorganic acids 16 acids 2 Brand. Sigma-Aldrich 16 Supelco 9 Boiling Point C Feature. Analytical 4 ACS reagent 2 BioReagent 2 Technique.
Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within. C60 Fullerene Buckminsterfullerene is a spherical shaped allotrope of carbon discovered in 1985. C70 Fullerene Fullerenes are spherical cagelike molecules consisting of annelated carbon five - and six rings.
Caffeine A stimulant found in drinks and used in pharmaceuticals. Calcite Calcite is the most common form of calcium carbonate. Calcium hydride Calcium Hydride is a cold-trapped molecule.
What is the difference between an inorganic compound and an organic compound. Organic compounds contain carbon and hydrogen sometimes in combination with other elements such as oxygen nitrogen sulfur and the halogens. Inorganic compounds generally do not contain carbon although some carbon-containing species are considered inorganic.
Write chemical formulas for the following molecular.