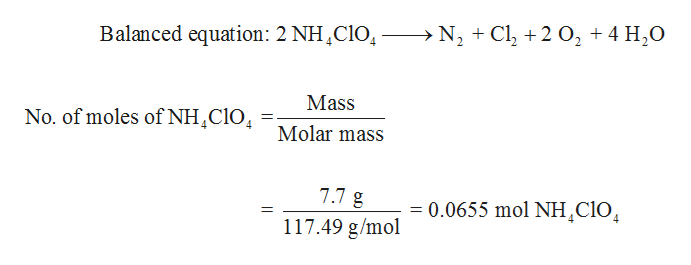
A short summary of this paper. Full PDF Package Download Full PDF Package.
Water vapors and gasses then shoot out of the rocket causing the.
Ammonium perchlorate chemical formula. Ammonium perchlorate AP is an inorganic compound with the formula NH 4 ClO 4. It is a colorless or white solid that is soluble in water. It is a powerful oxidizer.
Combined with a fuel it can be used as a rocket propellant. Its instability has involved it in a number of accidents such as the PEPCON disaster. Ammonium perchlorate an oxidizer is the most prevalent form of this compound.
Has been widely used in solid propellants fireworks and flares. And is a constituent of many munition components. Manufacture of ammonium perchlorate began in the 1940s primarily for use by the defense industry and later by the aerospace industry.
A perchlorate is a chemical compound containing the perchlorate ion ClO 4. The majority of perchlorates are commercially produced salts. They are mainly used as oxidizers for pyrotechnic devices and to control static electricity in food packaging.
Perchlorate contamination in food water and other parts of the environment has been studied in the US. Because of harmful effects on human. Is a member of a class of strong oxidizing agents.
May be self-reactive eg ammonium perchlorate glycol perchlorate and liable to violent decomposition. The violence of decomposition of some perchlorates exceeds that of nitroglycerine. Noncombustible but able to accelerate the burning of combustible materials.
NH42SO4 ammonium sulfate 20. NiS nickel II sulfide Write the chemical formula for each of the following compounds. Sodium nitrite NaNO2 31.
Potassium carbonate K2CO3 22. Iron III oxide Fe2O3 32. Silver sulfide Ag2S 23.
Aluminum hydroxide AlOH3 33. Nickel II carbonate NiCO3 24. Ammonium hydroxide NH4OH 34.
Calcium phosphate Ca3PO42 25. Magnesium chloride MgCl2 35. In this view dissociation seems more like a physical change than a chemical change but we can still represent the process as a chemical equation.
Of course just like any other chemical equation it must be balanced. The number of atoms of each type and the net charge on each side of the equation must be the same. So for example the dissociation equations for the soluble ionic.
Chemical Formula Nomenclature Practice. Complete these in lab and on your own time for practice. You should complete this by Sunday.
Use the stock form for the transition metals. Give the formula for the following. Sulfur dioxide SO2_ 2.
Sodium thiosulfate Na2S2O3_ 3. Nickel iii sulfide chemical formula. Long before chemists knew the formulas for chemical compounds they developed a system of nomenclature that gave each compound a unique name.
Today we often use chemical formulas such as NaCl C 12 H 22 O 11 and CoNH 3 6 ClO 4 3 to describe chemical compoundsBut we still need unique names that unambiguously identify each compound. Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more. Modification of work by vxlaFlickr.
Modification of work by the Italian voiceFlickr. Modification of work. Ammonium Bromide NH4Cl Ammonium Chloride NH4NO2 Ammonium Nitrite NH4NO3 Ammonium Nitrate NH4OH Ammonium Hydroxide NiNO32 NickelII Nitrate NI3 Nitrogen Triiodide NiBr2 Nickel Bromide NiCl2 NickelII Chloride NiO NickelII Oxide NiSO4 Nickel Sulfate NO2 Nitrogen Dioxide NO3 Nitrate Ion OF2 Oxygen Difluoride P2O3 Diphosphorus Trioxide P2O5.
Chemical compound - chemical compound - Binary molecular covalent compounds. Binary molecular covalent compounds are formed as the result of a reaction between two nonmetals. Although there are no ions in these compounds they are named in a similar manner to binary ionic compounds.
The nomenclature of binary covalent compounds follows these rules. These examples show how the rules. The information in a chemical formula.
How many moles and formulas are in 416 g ammonium carbonate. Skill 3-3 Mass Percent and the Chemical Formula 12 The formula shows the number of moles of each element. Use it to calculate the mass percent of each element on a mole basis.
The formula of the sugar glucose is C 6 H 12 O. Write each chemical formula and name each compound correctly. The instructor should also review the score for forming each compound.
Scoring is described in step 5 of the procedure. This activity is a game but it is also meant to be a teaching tool for proper naming procedures. Students will hopefully learn by repeatedly practicing naming simple compounds.
Instructors should emphasize team. Flash powder is an explosive used in all firecrackers and larger Salutes such as M-80s and Aerial Bombs. Although there are many different formulas for Flash Powder the safest and the industry standard is made just from potassium perchlorate and German Aluminum.
It is far superior to any other flash powder formula in many respects. In order to work together the aluminum and the ammonium perchlorate are held together by another compound called a binder. When mixed all together the fuel has a slightly rubbery consistency.
This rubbery substance is then packaged into a casing. As the fuel burns the heat and energy cause the inside of the rocket to heat up. Water vapors and gasses then shoot out of the rocket causing the.
The presence of a metal in a chemical formula indicates an ionic compound which is composed of positive ions cations and negative ions anions. A formula with only nonmetals indicates a molecular compound unless it is an ammonium NH 4 compound. Only ionic compounds are considered in this Tutorial.
There are tables of common ions in your lecture text p 56 cations and p 57 anions. Answer Key Ionic Molecular or an Acid Honors Chemistry Write which type of compound it is whether the compound is ionic molecular or an acid. If there is a multi-valent metal present write both the stock and classical name.
Formula Type Chemical Name CaO I Calcium oxide C 2 H 2 M Dicarbon dihydride LiOH I Lithium hydroxide SO 3 M Sulfur trioxide. The formula unit is the absolute grouping represented by the empirical formula of a compound either ionic or covalent. Empirical formulas are particularly useful for describing the composition of ionic compounds which do not contain readily identifiable molecules.
Some ionic compounds occur as hydrates which contain specific ratios of loosely bound water molecules called. This is the structure of an ammonium ion. Polyatomic ions with a positive 1 charge do occur but the main one youll encounter and need to know is the ammonium ion.
Remember because it is a cation when it reacts and forms a compound it is cited first in the chemical formula. Ammonium - NH 4 Polyatomic Ion Charge -1. This is the one of the resonant structures of.
Mixtures with any perchlorate can explode on impact ACS 146211-212. A mixture of damp sulfur and calcium hypochlorite produces a brilliant crimson flash with scatter of molten sulfur Chem. Takes fire spontaneously in chlorine dioxide and may produce an explosion Mellor 2289 1946-47.
Ignites if heated with chromic anhydride ignite and can explode Mellor 10. CrO 4 2-Hydrogen Carbonate or Bicarbonate. Cr 2 O 7 2-Anions from Organic Acids.
Harris Quantitative Chemical Analysis 8th edition. Full PDF Package Download Full PDF Package. A short summary of this paper.
7 Full PDFs related to this paper. Download Full PDF Package. In ordinary chemical reactions the nucleus of each atom and thus the identity of the element remains unchanged.
Electrons however can be added to atoms by transfer from other atoms lost by transfer to other atoms or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemistry of the elements. During the formation of some compounds atoms gain or lose.
NH 4 No interference for NH 4 500 ppm. Similar results are obtained for Ammonia. CO 3 2- No interference for CO 3 2- 500ppm using chloride resistant sensor.
ClO 3 No interference for ClO 3 500ppm. Cl No interference for Cl 200 ppm. COD reduced by up to 20 at Cl- levels of 500ppm using Chloride.