PS by USDA - As a color fixative and preservative wi or without sodium or potassium nitrate in the curing of red meat. Copper I bisulfite CuHSO3 43.

Nitrite is oxidized to nitrate by nitrite-oxidizing bacteria NOB.
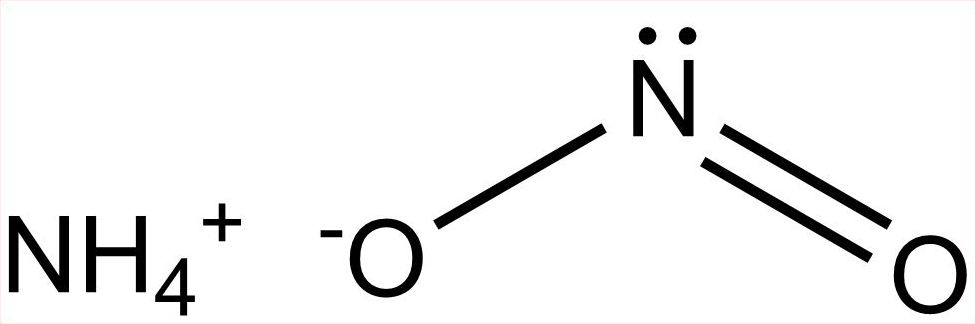
Ammonium nitrite formula. Ammonium nitrite forms naturally in the air and can be prepared by the absorption of equal parts nitrogen dioxide and nitric oxide in aqueous ammonia. 1 It can also be synthesized by oxidizing ammonia with ozone or hydrogen peroxide or in a precipitation reaction of barium or lead nitrite with ammonium sulfate or silver nitrite with ammonium chloride or ammonium perchlorate with. The ammonium cation is a positively charged polyatomic ion with the chemical formula NH 4.
It is formed by the protonation of ammonia NH 3. Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations NR 4 where one or more hydrogen atoms are replaced by organic groups indicated by R. What is the correct name for the ionic compound MnCO 3.
The formula unit for the ionic compound ammonium hydrogen sulfate consists of which of the following. 2 NH 4 ions and 1 SO 4 2-ion. 2 NH 4 ions and 1 HSO 4 2-ion.
1 NH 4-ion and 1 HSO 4 ion. 1 NH 4 ion and 1 HSO 4-ion. 1 NH 4 ion and 1 SO 4-ion.
Thereafter through a process called nitrification ammonia is oxidized to nitrite. Nitrite is oxidized to nitrate by nitrite-oxidizing bacteria NOB. It is both ammonium and nitrate which are the forms of nitrogen that are most readily absorbed by plants.
Urea Fertilizers Impact Soil pH. During the nitrification process there are increases in the number of free hydrogen ions H in the. Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box.
The names are found by finding the intersection between the cations and anions. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown. Zinc iron II iron III gallium silver lead IV chloride ZnCl 2.
Why and how did the ammonium nitrate cause this woman to appear blue-gray. Nitrogen is vital to all living things. The majority of the Earths atmosphere is made of nitrogen.
However plants and animals cannot make use of it in this gaseous form. It must first be converted to nitrates and nitrites. This is done through the nitrogen cycle in which microorganisms in soil and plant roots convert.
NH 4 NO 2. Ammonium oxide formula NH 4 2 O. Ammonium phosphate formula NH 4 3 PO 4.
Ammonium sulfate formula NH 4 2 SO 4. Ammonium sulfide formula NH 4 2 S. C 6 H 8 O 6.
BaC 2 H 3 O 2 2. Name to formula problems. 13 potassium fluoride is KF 14 ammonium sulfate is NH 42SO 4 15 magnesium iodide is MgI 2 16 copper II sulfite is CuSO 3 17 aluminum phosphate is AlPO 4 18 lead II nitrite is PbNO 22 19 cobalt II selenide is CoSe 20 silver cyanide is AgCN 21 copper II bicarbonate is CuHCO 32 22 iron II oxide.
Chemical formula plays an important role in understanding different concepts of chemistry. Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand. A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another.
Chemical formulas can be. Sodium nitrite - PRES REG 100 ppm to 200 ppm - In loin muscle of smoked chub - 172177. REG 10 ppm 0001 sodium nitrite - Alone as color fixative in smoked cured tuna -172175.
REG 20 ppm - In cnd pet food containing meat. PS by USDA - As a color fixative and preservative wi or without sodium or potassium nitrate in the curing of red meat. Answer 1 of 5.
When you get questions like this it helps a lot if you can see the general sorts of patterns. I will give the lowest level of explanation and then follow up with a high level explanation. In this case both of the reactants are salts.
They are each made of a ca. NH42SO4 ammonium sulfate 20. NiS nickel II sulfide Write the chemical formula for each of the following compounds.
Sodium nitrite NaNO2 31. Potassium carbonate K2CO3 22. Iron III oxide Fe2O3 32.
Silver sulfide Ag2S 23. Aluminum hydroxide AlOH3 33. Nickel II carbonate NiCO3.
Chemical Formula Nomenclature Practice. Complete these in lab and on your own time for practice. You should complete this by Sunday.
Use the stock form for the transition metals. Give the formula for the following. Sulfur dioxide SO2_ 2.
Sodium thiosulfate Na2S2O3_ 3. 4 Ammonium ion F Fluoride ion. 2 Nitrite ion.
Most Common Formula Representations All represent ethanol Example C 2H 6O CH 3CH 2OH Name Molecular Formula Condensed Molecular Formula Structural Formula Line Formula. Microsoft Word - Chem 1 Compound Sheet FS08 Author. Terry Bone Created Date.
922008 15513 PM. Structural formula indicates not only the number of atoms but also their arrangement in space. Let us consider a glucose molecule.
The molecular formula for glucose is C₆H₁₂O₆ which tells us the exact number of constituent atoms carbon hydrogen and oxygen written as C H and O respectively in one glucose molecule. Formula Oxidation State Organic nitrogen Org-N Ammonia NH 3-3 Ammonium ion NH 4 -3 Hydrazine N 2H 4-2 Hydroxylamine NH 2OH -1 Nitrogen gas N 2 0 Nitrous oxide N 2O 1 Nitric oxide NO 2 Nitrous acid HNO 2 3 Nitrite ion NO 2-3 Nitrogen dioxide NO 2 4 Nitric acid HNO 3 5 Nitrate ion NO 3-5 Nitrogen is of concern to agriculture both as an essential plant nutrient for building proteins and. Ammonification is the process by which microorganisms present in soil sediment or water mineralize low molecular weight dissolved organic molecules presenting amine or amide groups of general formula R-NH 2 and produce ammonium NH 4 Ammonification is the last step of the nitrogen cycle involving an organic compound and is the intermediary step between the depolymerization of large.
60 ammonium sulfate NH 4 2 SO 4 61 NaBr sodium bromide 62 CaC 2 H 3 O 2 2 calcium acetate 63 P 2 O 5 diphosphorus pentoxide 64 TiSO 4 2 titaniumIV sulfate 65 FePO 4 iron III phosphate 66 K 3 N potassium nitride 67 SO 2 sulfur dioxide 68 CuOH copper I hydroxide 69 ZnNO 2 2 zinc nitrite 70 V 2 S 3 vanadium III sulfide. In the first experiment fish n 360 were randomly distributed into separate aquariums at the density of 10 fish in each aquarium with expanding concentrations of sodium nitrite and ammonium chloride Merck Germany 100 110 120 130 140 150 mgL for ammonia and 450 500 550 600 650 700 mgL for nitrite for 96 h. All ammonia and nitrite exposure groups were conducted in.
Potassium nitrite KNO2 34. Ammonium hydroxide NH4OH 35. Magnesium phosphate Mg3PO42 36.
Copper II bisulfite CuHSO32 37. Tin II chlorate SnClO32 38. Calcium nitrate CaNO32 39.
Cesium permanganate CsMnO4 40. Silver dichromate Ag2Cr2O7 41. Ammonium phosphite NH43PO3 42.
Copper I bisulfite CuHSO3 43. Iron III acetate FeC2H3O23 44. Aluminum carbonate Al2CO33 45.
NH 3 In what follows the term ammonia covers both the nonionized form NH3 and the ammonium cation NH4 unless stated otherwise. Physicochemical properties 12 Property Value Melting point -7776 C Boiling point -3343 C Density of vapour 06 glitre at 20 C Water solubility 421 glitre at 20 C. 706 glitre at 0 C Vapour pressure 882 kPa at 20 C.
NH42S ammonium sulfide K3PO4 potassium phosphate ZnNO32 zinc nitrate Fe2SO43 ironIII sulfate or ferric sulfate CuCO3 copperII carbonate or cupric carbonate Note. In a formula parentheses are used around a polyatomic ion only when there are 2 or more of that polyatomic ion in a formula unit ie when the subscript is not 1. Name Formula Charge Name Formula Charge Name Formula Charge aluminum Al 3 3 magnesium Mg 2 2 carbonate CO 3 2 2 ammonium NH 4 1 manganese II Mn 2 2 chlorate ClO 3 1 barium Ba 2 2 manganese III Mn 3 3 chloride Cl 1 cadmium Cd 2 2 mercury I mercurous See note Hg 2 2 2 X 1 chromate CrO 4 2 2 calcium Ca 2 2 mercury II mercuric Hg 2 2.
Methyl nitrite has the molecular formula CH3NO2. All hydrogens are bonded to carbon and the order of atomic connections is CONO. Table 14 How to Write Lewis Structures Step 2.
Count the number of valence electrons. For a neutral molecule this is equal to the number of valence electrons of the constituent atoms. Table 14 How to Write Lewis Structures Step 2.
Count the number of. Students enrolled in Dr. Draganjacs Introduction to Chemistry CHEM1003 General Chemistry I CHEM1013 and General Chemistry II CHEM1023 classes are responsible for learning the names and formulae for the common acids and common reagents and for learning the names formulae and the charges for the common cations and anions listed below.