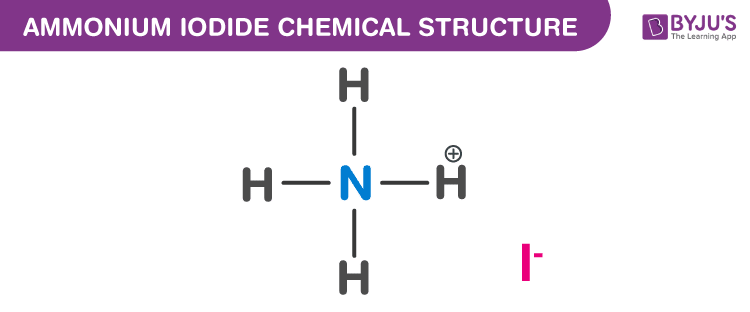
A chemical formula is a representation of a chemical substance using letters for atoms and subscript numbers to show the numbers of each type of atoms that are present in the substance. Ammonium iodide Identifiers CAS Number.

How many grams of NaCl are required to prepare 985 mL of 077 M NaCl solution.
Ammonium iodide molar mass. Jump to navigation Jump to search. Ammonium iodide Identifiers CAS Number. 14494 gmol Appearance White crystalline powder Density.
551 C 1024 F. 824 K Boiling point. 235 C 455 F.
508 K in vacuum Solubility in water. 155 g100 mL 0 C 172 g100 mL 20 C 250 g100 mL 100 C Magnetic susceptibility χ-660. Molar Mass of Frequently Calculated Chemicals.
C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4.
Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when. List of Iodide Compounds Common Compounds of Iodide I Formula Molecular Weight. Molecular Weight Molar Mass.
Fine white crystals or hygroscopic granules. 235 to 280 C. Preparation of Ammonium Sulfate NH42SO4.
Ammonium sulfate occurs naturally from the mineral mascagnite in volcanic fumaroles and due to coal fires on some dumps. Ammonium sulfate is formed by adding finely divided gypsum to. Ammonium Chloride NH 4 Cl- Ammonium chloride is an inorganic compound with formula NH 4 Cl.
In its pure form it is white crystalline salt. Ammonium chloride is used in veterinary medicine in the prevention of urinary stones in sheep goats and cattle. To learn more about the Structure Properties Preparation Uses and FAQs of Ammonium Chloride NH4Cl Visit BYJUS for more.
MV grams molar mass 00244234 molL 0100 L x 180155 gmol x 044 g Solution path 2. 1 Calculate how much glucose you have in 200 mL of the first solution. 110 g is to 100.
ML as x is to 200 mL Cross-multiply and divide 100x 110 times 200 x 22 g 2 When you dilute the 200 mL sample to 5000 mL you have 22 g glucose in the solution. 22 g is to 500. ML as x is to.
Chemical Reaction Formula Atomic Mass Formula Chemical Formula Enthalpy Formula Entropy Formula Molality Formula Molar Mass Formula Molarity Formula Structural Formula Molecular Formula Chemical Compound Formula Chemical Equilibrium Formula Normality Formula Photosynthesis Formula Grams to Moles Conversion Formula Moles to Grams Conversion Formula Radioactive Decay Formula. If the molar mass of the salt is 218 gmol what mass is required. What is the molarity of an aqueous solution of sodium hydroxide produced when 350 ml of a 540 M solution was diluted to 8900 ml.
How many grams of NaCl are required to prepare 985 mL of 077 M NaCl solution. What volume of 12 M HCl solution is needed to prepare 5 liters of 00250 M solution. How many grams of H_3PO_4.
Ammonium Ca2 calcium Fe2 ironII Cu2 copperII Mg2 magnesium Ni2 nickelII Sr2 strontium Zn2 zinc Sn2 tinII Pb2 leadII Ba2 barium Fe3 ironIII Al3 aluminium Cr3 ChromiumIII F-fluoride Cl-chloride Br-bromide I-iodide HO-hydroxide NO 2-nitrite NO 3-nitrate HCO 3-hydrogencarbonate O2-oxide CO 3 2-carbonate SO 4 2-sulfate HSO 4-hydrogensulfate S2-sulphide N3-nitride PO 4 3. The lead halide perovskite structure is represented by the general chemical formula APbX 3 where A denotes an organic ammonium or inorganic cation such as methylammonium MA formamidinium FA or Cs. And X denotes a halide I Br or Cl Corner-shared PbX 6 octahedra form a cuboctahedral cage to accommodate the A cation that satisfies the steric requirements and.
Cd 3 AsO 4 2. CdC 2 O 4 3H 2 O. Cd 3 PO 4 2.
How do you know the Order of Elements in a Chemical Formula What is Chemical Formula. A chemical formula is a representation of a chemical substance using letters for atoms and subscript numbers to show the numbers of each type of atoms that are present in the substance. Based on the chemical formula of a substance we know the composition of the substance.
We would like to show you a description here but the site wont allow us.