The hydrochloric acid would react violently with the ammonia to form ammonium chloride which is a very slightly acidic salt generally harmless. The safe ratio for this ammonia solution would be 2 tablespoons of ammonia ammonium hydroxide per half gallon of water.

Preparation of solutions calculator is a useful tool which allows you to calculate how many solid chemicals or stock solutions you will need to prepare the desired solution.
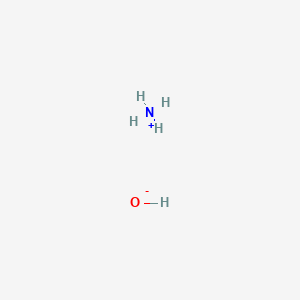
Ammonium hydroxide to ammonia. Ammonia solution also known as ammonia water ammonium hydroxide ammoniacal liquor ammonia liquor aqua ammonia aqueous ammonia or inaccurately ammonia is a solution of ammonia in water. It can be denoted by the symbols NH 3 aq. Although the name ammonium hydroxide suggests an alkali with composition NH 4 OH it is actually impossible to isolate samples of NH 4 OH.
Consumption pattern for ammonium hydroxide anhyd ammonia. Fertilizer use and chem int for fertilizer 74. Chem int in manufacture of fibers plastics resins and elastomers 6.
Chem int in manufacture of explosives 4. Chem int for livestock feed 2. Miscellaneous uses eg as a refrigerant as a solvent for casein and as a wood pulping chem 14 1975 sri.
Ammonium hydroxide commonly known as household ammonia is an ingredient in many household cleaning products used to clean a variety of surfaces including tubs sinks toilets countertops and tiles. Ammonia also is effective at breaking down household grime or stains from animal fats or vegetable oils such as cooking grease and wine stains. Because ammonia evaporates quickly it is.
Ammonium hydroxide solution with a 25 mass fraction has a melting point of 2157 K and a boiling point of 3108 K. Its standard enthalpy of formation is -80 Kilojoules per mole. Uses of Ammonium Hydroxide Ammonia water has many applications in industry as well as in laboratories.
Five such uses of ammonia solutions are listed below. The ammonium cation is a positively charged polyatomic ion with the chemical formula NH 4It is formed by the protonation of ammonia NH 3Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations NR 4 where one or more hydrogen atoms are replaced by organic groups indicated by R. Ammonium hydroxide is a colorless liquid chemical solution that is essentially ammonia dissolved in water.
Found in cleaning solutions its also used in much higher concentrations to kill dangerous bacteria such as salmonella and E. Different values of the pure water 0 concentration density reflect the fact that the measurements were done in different temperatures. Check our FAQ section for more details.
This is the list of all compounds with density tables present in the full database - it was actual on May 4 th 2005 as the list is growing it may be already incomplete. Acetic acid acetic aldehyde acetone. The reaction between Ammonium chloride and Barium hydroxide is an endothermic reaction.
BaOH 2 2NH 4 Cl BaCl 2 2NH 3 2H 2 O. Ammonium Chloride is slightly acidic and Barium Hydroxide very basic so will get an acid-base reaction. This reaction is used to recover ammonia in the preparation of sodium carbonate from NaCl.
Endothermic reactions are chemical. Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses.
Ammonia readily dissolves in water with the liberation of heat. NH 3 H 2 O NH 4 OH These aqueous solutions of ammonia are basic and are sometimes called solutions of ammonium hydroxide NH 4 OH. The equilibrium however is such that a 10-molar solution of NH 3 provides only 42 millimoles of hydroxide ion.
Preparation of NH4OH solution. Preparation of solutions calculator is a useful tool which allows you to calculate how many solid chemicals or stock solutions you will need to prepare the desired solution. When concentrated ammonia solution ammonium hydroxide is added to a clear light blue aqueous solution of copperII chloride a powdery light blue precipitate of copperII hydroxide forms.
Further addition of ammonia causes the copper ion to go back into solution as a deep blue ammonia complex. The addition of 12M sulfuric acid reverses the changes through the copper hydroxide precipate. The exact proportion of ammonium chloride recovered depends on the relative demands for sodium carbonate and ammonium chloride.
If economic conditions require part of the ammonia can be recovered and returned to the brine -ammoniation step by distillation of the ammonium chloride solution in the presence of lime. Quaternary Ammonium Compounds Quats Quaternary ammonium compounds also called quats or QACs are a group of chemicals used for a variety of purposes including as preservatives surfactants antistatic agents and as active ingredients in disinfectants and sanitizers. Quats can be highly effective at killing bacteria fungi and viruses including SARS-CoV-2 the virus that causes COVID-19.
Ammonia interacts immediately upon contact with available moisture in the skin eyes oral cavity respiratory tract and particularly mucous surfaces to form the very caustic ammonium hydroxide. Ammonium hydroxide causes the necrosis of tissues through disruption of cell membrane lipids saponification leading to cellular destruction. As cell proteins break down water is extracted.
The safe ratio for this ammonia solution would be 2 tablespoons of ammonia ammonium hydroxide per half gallon of water. If youre worried about the dangers of cleaning with ammonia you can opt to clean your windows with vinegar. As weve already mentioned ammonia is relatively streak free so its a great alternative to get rid of fingerprints on mirrors.
You can take a look at more. As you know ammonia acts as a weak base in aqueous solution so right from the start you should expect the pH of the solution to be 7. NH_ 3aq H_ 2O_ l rightleftharpoons NH_ 4aq OH_ aq- The ratio that exists between the equilibrium concentrations of the ammonium cations and of the hydroxide anions and the equilibrium concentration of ammonia is.
Answer 1 of 16. I assume you mean an aqueous solution of ammonia which is an equilibrium system of NH4OH ammonium hydroxide with NH3 ammonia gas. The hydrochloric acid would react violently with the ammonia to form ammonium chloride which is a very slightly acidic salt generally harmless.
0215 external icon AMMONIUM HYDROXIDE 10-35 solution Aqua ammonia Ammonium hydrate 0414 external icon AMMONIA ANHYDROUS R717 Refrigerant gas 717 1051 external icon AMMONIUM CHLORIDE Sal ammoniac 1234 external icon NITROGEN TRIFLUORIDE Nitrogen fluoride Trifluoroamine Trifluoroammonia Perfluoroammonia INCHEM Health and Safety Guide No. Major products include anhydrous ammonia NH 3 and aqua ammonia ammonium hydroxide NH 4 OH. We are also a leading supplier of other chemicals including methylamines a derivative of ammonia and hydrogen chloride HCl.
Our anhydrous ammonia aqua ammonia hydrogen chloride and methylamine products are guaranteed to meet industry leading specifications. A 25 aqueous ammonia solution is as the name implies 25 by weight ammonia not ammonium hydroxide. It is true that this is often called ammonium hydroxide.
In fact the solution only contains a. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. This is our newest publication and has been created to support the school technician profession in Scotland.
Ammonia includes anhydrous ammonia and aqueous ammonia from water dissociable ammonium salts and other sources. 10 percent of total aqueous ammonia is reportable under this listing 7664-41-7. 313 X indicates that this is a second name for an EPCRA section 313 chemical already included on this consolidated list.
May also indicate that the. Ammonia NH is a colorless irritant gas with a pungent order that is readily soluble in water to generate ammonium NH ions1 Ammonia is a natural by-product in the human body as an intermediate in several metabolic reactions primarily involving amino acid synthesis2 It also gets produced in the human gut as a result of various enzymatic actions of bacteria3 However as a result. Ammonia is a weak base and forms a few ammonium and hydroxide ions in solution.
NH 3 g H 2 Ol NH 4 aq OH aq The hexa-aqua-copperII ions react with hydroxide ions to form a precipitate. This involves deprotonation of two of the water ligand molecules. CuH 2 O 6 2 aqpale blue 2OH aq CuH 2 O 4 OH 2spale blue precipitate 2H 2 Ol The copper.