
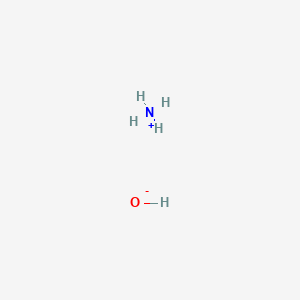
Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box. The general structure of an NH 4 OH molecule is illustrated above.
1 NaBr sodium bromide 2 ScOH3 scandium hydroxide 3 V2SO43 vanadium III sulfate 4 NH4F ammonium fluoride 5 CaCO3 calcium carbonate 6 NiPO4 nickel III phosphate 7 Li2SO3 lithium sulfite 8 Zn3P2 zinc phosphide 9 SrC2H3O22 trontium acetates.
Ammonium hydroxide formula. Ammonium hydroxide with more than 10 but not more than 35 Ammonia US. 2004 Emergency Response Guidebook. A Guide book for First Responders During the Initial Phase of a Dangerous GoodsHazardous Materials Incident.
Ammonia solution also known as ammonia water ammonium hydroxide ammoniacal liquor ammonia liquor aqua ammonia aqueous ammonia or inaccurately ammonia is a solution of ammonia in water. It can be denoted by the symbols NH 3 aq. Although the name ammonium hydroxide suggests an alkali with composition NH 4 OH it is actually impossible to isolate samples of NH 4 OH.
What is NH 4 OH Ammonium Hydroxide. NH 4 OH is the chemical formula of a solution of ammonia in water. This solution is known by various names such as ammonia water ammonium hydroxide ammonia liquor and aqueous ammonia.
NH 4 OH is often denoted by the symbol NH 3 aq. The general structure of an NH 4 OH molecule is illustrated above. Basicity of NH 4 OH.
Ammonium sulfide also known as the stink bomb is a toxic chemical and has a very strong and unpleasant odour. They are highly flammable and the ammonium sulfide molecular formula is represented as follows NH4 2 S. Treating ammonium hydroxide with an excess of hydrogen sulfide ammonium hydrosulfide NH 4 HS is formed which with further treatment with the same quantity of.
The ammonium cation is a positively charged polyatomic ion with the chemical formula NH 4It is formed by the protonation of ammonia NH 3Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations NR 4 where one or more hydrogen atoms are replaced by organic groups indicated by R. Ionic Compound Naming and Formula Writing List 1. Copy this to my account.
E-mail to a friend. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Polyatomic ions are ions which consist of more than one atom.
For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atomsThe atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit. The molecular formula for glucose is C₆H₁₂O₆ which tells us the exact number of constituent atoms carbon hydrogen and oxygen written as C H and O respectively in one glucose molecule. The empirical formula of glucose is CH₂O which represents the whole number ratio of the constituent atoms viz C H.
Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box. The names are found by finding the intersection between the cations and anions. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
Zinc iron II iron III gallium silver lead IV chloride ZnCl 2. Ferric ammonium citrate iron III ammonium citrate is prepared by the reaction of ferric hydroxide with citric acid followed by treatment with ammonium hydroxide evaporating and drying. The resulting product occurs in two forms depending on the stoichiometry of the initial reactants.
52 ammonium oxide NH 4 2 O 33 potassium hydroxide KOH 54 lead IV sulfate PbSO 4 2 55 silver cyanide AgCN 56 vanadium V nitride V 3 N 5 57 strontium acetate SrC 2 H 3 O 2 2 58 molybdenum VI sulfate MoSO 4 3 59 platinum II sulfide PtS 60 ammonium sulfate NH 4 2 SO 4 61 NaBr sodium bromide 62 CaC 2 H 3 O 2 2 calcium. Formula writing rules to write the correct chemical formulas for each compound. Compound Name Type of Compound.
Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate. Solubility Product Constants near 25 C. Ionic Compound Formula K sp.
Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3. Name Formula Charge Name Formula Charge Name Formula Charge aluminum Al 3 3 magnesium Mg 2 2 carbonate CO 3 2 2 ammonium NH 4 1 manganese II Mn 2 2 chlorate ClO 3 1 barium Ba 2 2 manganese III Mn 3 3 chloride Cl 1 cadmium Cd 2 2 mercury I mercurous See note Hg 2 2 2 X 1 chromate CrO 4 2 2 calcium Ca 2 2 mercury II mercuric Hg 2 2. Ionic Naming Practice Problems - Solutions Name the following ionic compounds.
1 NaBr sodium bromide 2 ScOH3 scandium hydroxide 3 V2SO43 vanadium III sulfate 4 NH4F ammonium fluoride 5 CaCO3 calcium carbonate 6 NiPO4 nickel III phosphate 7 Li2SO3 lithium sulfite 8 Zn3P2 zinc phosphide 9 SrC2H3O22 trontium acetates. In chemistry a base is a chemical species that donates electrons accepts protons or releases hydroxide OH- ions in aqueous solution. Bases display certain characteristic properties that can be used to help identify them.
They tend to be slippery to the touch eg soap can taste bitter react with acids to form salts and catalyze certain reactions. Types of bases include Arrhenius.