Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. To learn more about the preparation physical and chemical properties Uses and FAQs of Ammonium Nitrate VIsit BYJUS for more content.
Some acids are strong electrolytes because they ionize completely in water yielding a great many ions.
Ammonium hydroxide chemical properties. Ammonium hydroxide NH4OH or H5NO CID 14923 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. Ammonium Hydroxide NH4OH - NH4OH Ammonium Hydroxide is the Chemical Formula of a Solution of Ammonia in Water.
Learn about the structure properties. Ammonia a colorless gas with a distinct odor is a building-block chemical and a key component in the manufacture of many products people use every day. It occurs naturally throughout the environment in the air soil and water and in plants and animals including humans.
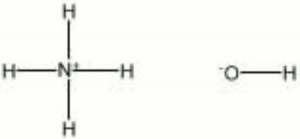
The human body makes ammonia when the body breaks down foods containing protein into. Ammonia solution also known as ammonia water ammonium hydroxide ammoniacal liquor ammonia liquor aqua ammonia aqueous ammonia or inaccurately ammonia is a solution of ammonia in water. It can be denoted by the symbols NH 3 aq.
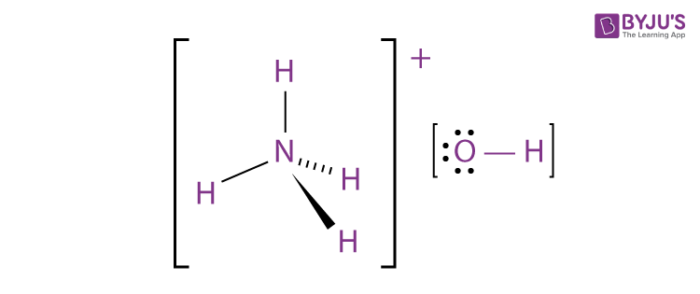
Although the name ammonium hydroxide suggests an alkali with composition NH 4 OH it is actually impossible to isolate samples of NH 4 OH. The ammonium cation is a positively charged polyatomic ion with the chemical formula NH 4. It is formed by the protonation of ammonia NH 3.
Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations NR 4 where one or more hydrogen atoms are replaced by organic groups indicated by R. The acid-forming properties of ammonium chloride result from dissociation of the salt to an ammonium cation and a chloride anion. In patients with normal hepatic function the ammonium cation is converted to urea by the liver and a hydrogen cation is released which reacts with a bicarbonate ion to form water and carbon dioxide.
Ammonium NitrateNH 4 NO 3 -Ammonium Nitrate is an Ionic Salt Made up of the Ammonium Cation NH 4 and the Nitrate Anion NO 3 -. It is white crystalline solid used as a key component of many fertilizers. To learn more about the preparation physical and chemical properties Uses and FAQs of Ammonium Nitrate VIsit BYJUS for more content.
Ammonium Fluoride 20 180 Ammonium Hydroxide 180 Ammonium Nitrate 180 Ammonium Persulfate NR Ammonium Phosphate 180 Dibasic 180 Monobasic 180 Tribasic 180 Ammonium Sulfate 180 Ammonium Sulfide 180 Amyl Acetate NR Amyl Chloride NR Aniline 150 Aniline Hydrochloride 73 Anthraquinone Sulfonic Acid 73 Anti-Freeze See Ethylene Glycol 150 Antimony Trichloride 120 Aqua. A chemical formula is an expression that represents the element in that compound along with its relative proportion in the compound. Chemical formula for water is H₂O which means that water is a compound which is formed by the combination of 2 proportions of H and 1 proportion of O.
Similarly sulfuric acid has a chemical formula H₂SO₄ which means that in this compound the. Polyethylene Chemical Compatibility Guide Page 1 of 5 Chemical Compatibility Guide For Polyethylene Items This Chemical Compatibility Guide is offered for informational purposes only and was developed from information sources other than SpillTech. The information from such third party sources is believed to be reliable and accurate.
However Purchaser should make its own determination of. The Physical Property fields include properties such as vapor pressure and boiling point as well. Ammonia includes anhydrous ammonia and aqueous ammonia from water dissociable ammonium salts and other sources.
10 percent of total aqueous ammonia is reportable under this listing 7664-41-7. 313 X indicates that this is a second name for an. Ammonium hydroxide 3 molar.
The usual range of pH values encountered is between 0 and 14 with 0 being the value for concentrated hydrochloric acid 1 M HCl 7 the value for pure water neutral pH and 14 being the value for concentrated sodium hydroxide 1 M NaOH. It is possible to get a pH of -1 with 10 M HCl but that is about a practical limit of acidity. At the other extreme a 10 M solution of NaOH would have a.
We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. This is our newest publication and has been created to support the school technician profession in Scotland. Acids are a distinct class of compounds because of the properties of their aqueous solutions.
Those properties are outlined below. Aqueous solutions of acids are electrolytes meaning that they conduct an electrical current. Some acids are strong electrolytes because they ionize completely in water yielding a great many ions.
Other acids are weak electrolytes that exist primarily in a non. Ferrics Alums and Polymers. Fats Oils and Grease.
Sloped Bottom Vertical Tanks with IMFO High-Density Cross-linked Polyethylene XLPE NSFANSI 61 Certification. Write balanced chemical equations for the acid-base reactions described here. A the weak acid hydrogen hypochlorite reacts with water b a solution of barium hydroxide is neutralized with a solution of nitric acid.
Solution a The two reactants are provided HOCl and H 2 O. Magnesium hydroxide is an inorganic compound. It is naturally found as the mineral brucite.
Magnesium hydroxide can be used as an antacid or a laxative in either an oral liquid suspension or chewable tablet form. Additionally magnesium hydroxide has smoke suppressing and flame retardant properties and is thus used commercially as a fire.