
In place of the caustic solution used to dissolve the lignin in the wood sulfurous acid is employed. Aluminum dichromate Al2Cr2O73 14.
Water Hydrogen sulfate Hydrogen phosphate Hydrogen nitrate RevisedST72913.
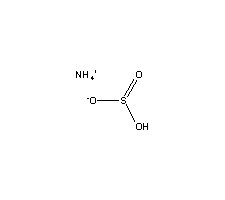
Ammonium hydrogen sulfite. List of Hydrogen Sulfite Compounds Common Compounds of Hydrogen Sulfite HSO3 Formula Molecular Weight. The ammonium cation is a positively charged polyatomic ion with the chemical formula NH 4. It is formed by the protonation of ammonia NH 3.
Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations NR 4 where one or more hydrogen atoms are replaced by organic groups indicated by R. Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses.
The hydrogen sulfide producing bacteria H 2 S comprise various groups of bacteria and archaea that obtain energy by reducing various compounds that possess sulfur in their molecule including organic compounds sulfur amino acids and inorganic compounds with oxidized sulfur such as sulfate sulfite thiosulfate tetrathionates or elemental sulfur to H 2 S. Hydrogen sulfide is a. Polyatomic ions are ions which consist of more than one atom.
For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atomsThe atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
The main target users are workers and those responsible for occupational safety and health. The ICSC project is a common undertaking between the World Health Organization WHO and. The production of acid sulfite pulp proceeds similarly to kraft pulping except that different chemicals are used in the cooking liquor.
In place of the caustic solution used to dissolve the lignin in the wood sulfurous acid is employed. To buffer the cooking solution a bisulfite of sodium magnesium calcium or ammonium is used. We therefore need a series of rules that allow us to unambiguously name positive and negative ions before we can name the salts these ions form.
Monatomic positive ions have the name of the element from which they are formed. Na sodium. Zn 2 zinc.
Ca 2 calcium. H hydrogen. K potassium.
Sr 2 strontium. Ammonium sulfate is an inorganic sulfate salt obtained by reaction of sulfuric acid with two equivalents of ammoniaA high-melting decomposes above 280 white solid which is very soluble in water 706 g100 g water at 0. 1038 g100 g water at 100 it is widely used as a fertilizer for alkaline soils.
It has a role as a fertilizer. Ammonium chloride Ammonium carbonate Ammonium hydroxide Ammonium sulfate Ammonium. Hydrogen chloride Hydrogen carbonate.
Water Hydrogen sulfate Hydrogen phosphate Hydrogen nitrate RevisedST72913. Department of Chemistry University of Texas at Austin I. Name the following Ionic Compounds.
LiBr lithium bromide. NH 4 NH 3. H 3 BO 3.
H 2 BO 3-Dihydrogen carbonate ion. Hydrogen carbonate ion. HCO 3-CO 3 2-Carbonate ion.
Hydrogen phosphate ion. HPO 4 2-PO 4 3. Group ADI expressed as sulfur dioxide for calcium hydrogen sulfite calcium metabisulfite calcium sulfite potassium hydrogen sulfite.
Nair B Elmore AR. Final report on the safety assessment of sodium sulfite potassium sulfite ammonium sulfite sodium bisulfite ammonium bisulfite sodium metabisulfite and potassium metabisulfite. Int J Toxicol 22 Suppl 2 63-88 2003 Cosmetic.
NH 4 nitrite. Bisulfate HSO 4. S 2 O 3 2.
C 2 O 4 2. H 2 PO 4. In the presence of ammonium compounds ammonium hydroxide ammonium carbonate ammonium hydrogen carbonate and ammonium phosphate.
No sulfite compounds are used. With both sulfite and ammonia reactants. Also called sulfite ammonia caramel.
In the presence of both sulfite and ammonium compounds. What is Caramel Color made of. As caramelization is a.
Ammonium acetate S S Boric acid dilute S S Ammonium bromide S S Brine S S Ammonium carbonate S S Bromic acid 10 S S Ammonium chloride saturated S S Bromine liquid 100 O U Ammonium fluoride 20 S S Bromochloromethane U U Ammonium hydroxide S S Butadiene U U Ammonium metaphosphate sat S S Butanediol 10 S S. Ammonium NH4 acetate CH3COO-C2H3O2-bromate BrO3-carbonate CO3 2-chlorate ClO3-chlorite ClO2-chromate CrO4 2-cyanide CN-dichromate Cr2O7 2-hydrogen carbonate bicarbonate HCO3-hydrogen sulfate bisulfate HSO4-hydrogen phosphate biphosphate HPO4 2-hydroxide OH-hypochlorite ClO-iodate IO3-nitrate NO3-nitrite NO2-oxalate C2O4 2-perchlorate ClO4-periodate IO4-permanganate MnO4. Ammonium NH 4 1 manganese II.
Hydrogen carbonate HCO 3 1 sulfite SO 2 2 lithium Li 1 bisulfate hydrogen sulfate HSO 4 1 sulfide S 2 2 nickelII nickelous Ni 2 2 bromate BrO 3 1 thiocyanate SCN 1 potassium K 1 bromide Br 1 thiosulfate S 2 O 3 2 2 A note about hydrogen and hydronium. Rarely does hydrogen ion exist on its. Common Covalent Binary Inorganic Compounds of atoms Prefix element closest to fluorine goes on rightCommon Examples 1 Mono H 2 Hydrogen N 2 Nitrogen 2 Di O 2 Oxygen NH 3 Ammonia 3 Tri O 3 Ozone NO Nitrogen monoxide Nitric Oxide 4 Tetra H 2O Water Dihydrogen Monoxide NO 2 Nitrogen dioxide 5 Penta F 2 Fluorine N 2O Dinitrogen monoxide Nitrous oxide 6 Hexa HF Hydrogen fluoride N.
HPO 4 2-Dihydrogen phosphate. H 2 PO 4-Sulfate. S 2 O 3 2-Sulfite.
CrO 4 2-Hydrogen carbonate or. The first number under the column headed RQ is the reportable quantity in pounds. The number in parentheses is the metric equivalent in kilograms.
Hydrogen peroxide is a chemical compound with the formula H 2 O 2In its pure form it is a very pale blue liquid slightly more viscous than waterIt is used as an oxidizer bleaching agent and antiseptic usually as a dilute solution 36 by weight in water for consumer use and in higher concentrations for industrial useConcentrated hydrogen peroxide or high-test peroxide. Ammonium NH 4 nitrate NO 3 acetate C 2 H 3 O 2 or CH 3 COO hydrogen carbonate HCO 3 chlorate ClO 3 halides Cl Br I when combined with Ag Pb2 or Hg 2 2 sulfates SO 4 2 when combined with Ag Ca2 Sr2 Ba2 or Pb2 Ions That Form Insoluble Compounds Exceptions carbonate CO 3 2 when combined with Group 1 ions or ammonium NH 4. Corrosion - Corrosion in piping systems - caused by thermodynamic and electrochemical processes - corrosion problems and methods of protection and prevention.
Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. Chemical Resistance of Rubbers and Elastomers - Rsistance to chemicals. Ammonium NH 4 cesium Cs goldI Au hydrogen.
Hydrogen sulfate hydroxide OH-bisulfate HSO 4 -. Sulfite SO 3 2-tartrate C 4 H 4 O 6 2-tetraborate B 4 O 7 2-thiosulfate S. Ammonium cyanide NH4CN 5.
Cadmium phosphate Cd3PO42 9. Tin II bicarbonate SnHCO32 10. Potassium nitrate KNO3 11.
Mercury I cyanide Hg2CN2 12. Iron II acetate FeC2H3O22 13. Aluminum dichromate Al2Cr2O73 14.
Silver sulfate Ag2SO4 15. Tin IV nitrite SnNO24 16. Strontium chlorate SrClO32 17.
Cesium hydrogen sulfite CsHSO 3 18.