See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. COD is used to gauge the short-term impact wastewater effluents will have on the oxygen levels of receiving waters.
Dental caries can result in loss of tooth structure pain and tooth loss and can progress to acute systemic infection.
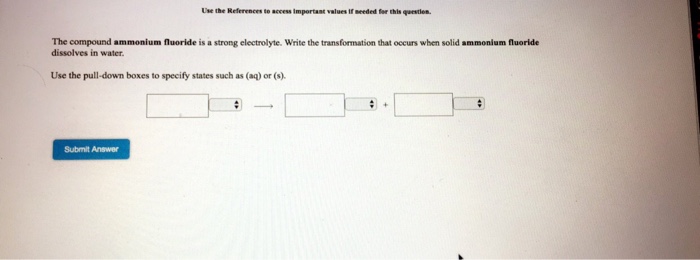
Ammonium fluoride dissolved in water. When ammonia is dissolved in water a tiny amount of it converts to ammonium ions. H 2 O NH 3 OH NH 4. The degree to which ammonia forms the ammonium ion depends on the pH of the solution.
If the pH is low the equilibrium shifts to the right. More ammonia molecules are converted into ammonium ions. If the pH is high the concentration of hydrogen ions is low the equilibrium.
The 100 air saturation means that the water is holding as many dissolved gas molecules at it can in equilibrium. At equilibrium the percentage of each gas in the water would be equivalent to the percentage of that gas in the atmosphere which is known as its partial pressure. The water will slowly absorb oxygen and other gases from the atmosphere until it reaches equilibrium at complete.
Dissolved solids smaller than 2 microns refer to any minerals salts metals in the form of molecules atoms cations or anions dissolved in water. Total dissolved solids TDS comprise inorganic salts principally calcium magnesium potassium sodium bicarbonates chlorides and sulfates and some small amounts of organic matter that dissolve in water. Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes.
We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. Water testing equipment and supplies. Acidity Alkalinity Aluminium Ammonia Ammonium Anionic Surfactants Boron Bromide Bromine Cadmium Calcium.
Carbon Dioxide Chloride Chlorine Chlorine Dioxide Chromium COD Colour Conductivity TDS Copper Cyanide Cyanuric Acid. Dissolved Oxygen Formaldehyde Fluoride Glycol Hardness Hydrazine Hydrogen Peroxide Hypochlorite Iodide Iodine Iron. Lead Lux Magnesium Manganese Molybdenum.
Fluoride toxicity is a condition in which there are elevated levels of the fluoride ion in the body. Although fluoride is safe for dental health at low concentrations sustained consumption of large amounts of soluble fluoride salts is dangerous. Referring to a common salt of fluoride sodium fluoride NaF the lethal dose for most adult humans is estimated at 5 to 10 g which is equivalent.
This solution is known by various names such as ammonia water ammonium hydroxide ammonia liquor and aqueous ammonia. NH 4 OH is often denoted by the symbol NH 3 aq. The general structure of an NH 4 OH molecule is illustrated above.
Basicity of NH 4 OH. When ammonia is dissolved in water the water molecules donate a proton to the NH 3 molecule. This leads to the formation of an.
The CA610 Fluoride Analyzer uses advanced ion-selective electrode ISE technology for continuous monitoring of fluoride concentration in drinking water effluent. Accuracy is ensured with precise control of temperature. Standards for Water Quality Sampling ASNZS 566711998 and methods described by the Standard methods for the examination of water and waste water American Public Health Association APHA 1998.
This publication is designed to provide accurate standardised methodology for those involved in developing water quality monitoring programs. Ionic bonds are atomic bonds created by the attraction of two differently charged ionsThe bond is typically between a metal and a non-metal. The structure of the bond is rigid strong and often crystalline and solid.
Ionic bonds also melt at high temperatures. When solid sodium fluoride is dissolved into water it completely dissociates into sodium ions and fluoride ions. The sodium ions do not have any capability of hydrolyzing but the fluoride ions hydrolyze to produce a small amount of hydrofluoric acid and hydroxide ion.
Salts that are derived from the neutralization of a weak acid HF by a strong base NaOH will always produce salt solutions. The amount of purified water delivered in a given time and the degree of salts removed depends on the pressure of the system membrane type total dissolved solids of the water being purified and temperature. Efficiency is strongly dependent on the integrity and cleanliness of the membranes.
Chlorine can cause rapid degradation of the membranes and sediments cause clogging. For this reason. Ammonium ion NH 4 -3 Hydrazine N 2H 4-2 Hydroxylamine NH 2OH -1 Nitrogen gas N 2 0 Nitrous oxide N 2O 1 Nitric oxide NO 2 Nitrous acid HNO 2 3 Nitrite ion NO 2-3 Nitrogen dioxide NO 2 4 Nitric acid HNO 3 5 Nitrate ion NO 3-5 Nitrogen is of concern to agriculture both as an essential plant nutrient for building proteins and amino acids and as a potential water pollutant.
High Purity Water Feedwater Steam. Chlorine Ozone Chlorine dioxide. Hydrazine Carbohydrazide.
Organics UV254 Oxygen dissolved pH. Redox ORP Silica. Total Organic Carbon.
The first use of adjusted fluoride in water for caries control began in. Acids dissolve the hard surfaces of teeth. Unchecked the bacteria can penetrate the dissolved surface attack the underlying dentin and reach the soft pulp tissue.
Dental caries can result in loss of tooth structure pain and tooth loss and can progress to acute systemic infection. Cariogenic bacteria ie. What hydrogen fluoride is.
Hydrogen fluoride is a chemical compound that contains fluorine. It can exist as a colorless gas or as a fuming liquid or it can be dissolved in water. When hydrogen fluoride is dissolved in water it may be called hydrofluoric acid.
Hydrogen fluoride can be released when other fluoride-containing compounds such as ammonium fluoride are combined with water. When dissolved in water acidic salts will yield solutions with pH less than 70. This is due either to the presence of a metal cation that acts as a Lewis acid which will be discussed in a later concept or quite commonly due to a hydrolyzable proton in the cation or the anion.
Salts with acidic protons in the cation are most commonly ammonium salts or organic compounds that contain a. Published since 1928 Water Environment Research WER is an international multidisciplinary water resource management journal for the dissemination of fundamental and applied research in all scientific and technical areas related to water quality and resource recovery. WERs goal is to foster communication and interdisciplinary research between water sciences and related fields such as.
Chemical oxygen demand COD is the amount of dissolved oxygen that must be present in water to oxidize chemical organic materials like petroleum. COD is used to gauge the short-term impact wastewater effluents will have on the oxygen levels of receiving waters. Water Quality Guidelines for the Protection of Aquatic Life Freshwater Marine.
Water Quality Guidelines for the Protection of Agriculture Irrigation Livestock. Sediment Quality Guidelines for the Protection of Aquatic Life Freshwater and Marine ISQGPEL. Soil Quality Guidelines for the Protection of Environmental and Human Health Agricultural ResidentialParkland Commercial Industrial.
Hydrogen fluoride mixes readily with water forming hydrofluoric acid. For all practical purposes they are considered the same chemical. Hydrogen fluoridehydrofluoric acid is used extensively in the extraction processing and refining of metals rock brick and oil.
It is an intermediate for many chemical reactions and syntheses. It is used to remove and inhibit rust and to etch polish. Dissolved solids are reported as a concentration value in mgL milligrams per liter calculated by summing all major ions.
Dissolved-solids concentrations often are used to compare water quality between different groups of water samples. Dissolved-solids concentrations were used to compare data collected as part of this study to data collected by several other investigations with larger. Boiling point - the temperature at which a liquid turns into a gas.
Melting point - the temperature at which a solid turns into a liquid. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
Naming Salts Ionic Compounds Salts are ionic compounds which when dissolved in water break up completely into ions. They arise by the reaction of acids with bases and they always contain either a metal cation or a cation derived from ammonium NH 4. Examples of salts include NaCl NH 4 F MgCO 3 and Fe 2 HPO 4 3.
Salts are named by listing the names of their component ions cation.