BaCl 2 K 2 SO 4 BaSO 4 2 KCl By examining the solubility rules we see that while most sulfates are soluble barium sulfate is not. Ammonium chloride is an inorganic compound with the formula NH 4 Cl and a white crystalline salt that is highly soluble in water.

They are each made of a ca.
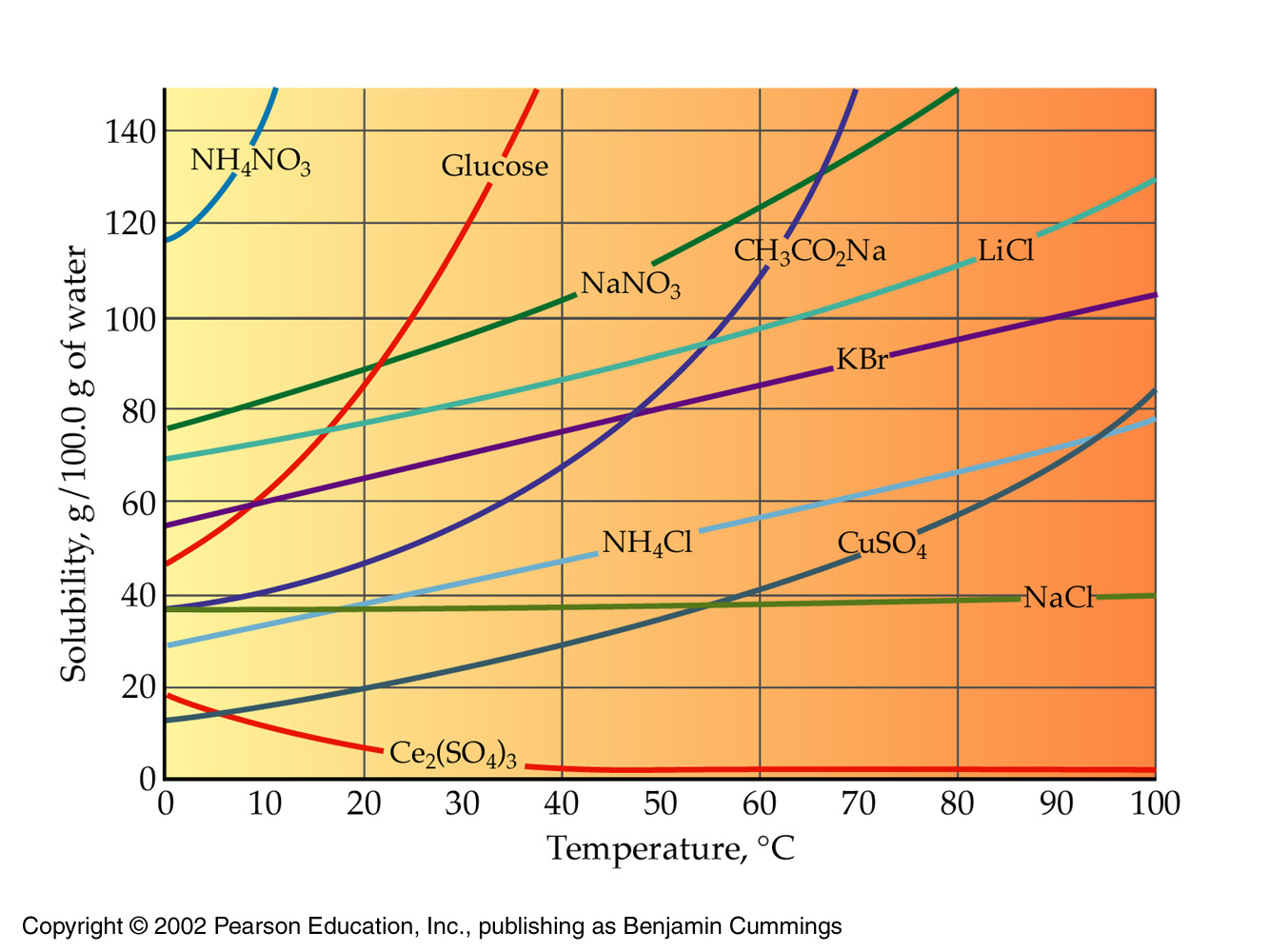
Ammonium chloride water solubility. Ammonium chloride is an inorganic compound with the formula NH 4 Cl and a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. In its naturally occurring mineralogic form it is known as sal ammoniacThe mineral is commonly formed on burning coal dumps from condensation of coal-derived gases.
It is also found around some types of volcanic vents. For more Solubility Complete data for AMMONIUM CHLORIDE 10 total please visit the HSDB record page. Hazardous Substances Data Bank HSDB Solubility in water g100ml at 25 C.
ILO International Chemical Safety Cards ICSC 37. The National Institute for Occupational Safety and Health NIOSH 328 Density. 153 at 68 F USCG 1999 US.
372 g100ml 20 C Ethanol. 2 g100ml Log Pow. -437 Estimated value Auto-ignition temperature.
400 C EU Method A16. Relative Self-Ignition Temperature for Solids Ammonium Chloride Safety Data Sheet according to Federal Register Vol. 58 Monday March 26 2012 Rules.
Ammonium cation is found in a variety of salts such as ammonium carbonate ammonium chloride and ammonium nitrateMost simple ammonium salts are very soluble in water. An exception is ammonium hexachloroplatinate the formation of which was once used as a test for ammoniumThe ammonium salts of nitrate and especially perchlorate are highly explosive in these cases ammonium is the reducing. Answer 1 of 5.
When you get questions like this it helps a lot if you can see the general sorts of patterns. I will give the lowest level of explanation and then follow up with a high level explanation. In this case both of the reactants are salts.
They are each made of a ca. Didecyl dimethyl ammonium chlorides production and use as a disinfectant and microbiocide in various applications may result in its release to the environment through various waste streams. Its use as a general purpose disinfectant and pesticide in water treatment of cooling towers and as a wood preservative will result in its direct release to the environment.
If released to air a vapor. Solubility and solubility product are good points to understand the solubility of a compound and they can be used to AgCl too. Solubility of silver chloride in water.
Solubility of AgCl is 520 µg100 g of water at 50 0 C. So it is a very low value and prove furthermore AgCl is a. The active agent 3-trimethoxysilylpropyl dimethyloctadecyl ammonium chloride.
Ledbetter and Bowen 5 estimated that the CMC of SMBA-Cl was about 39 mgL which can be taken as a rough approximation of water solubility. The most significant mechanism for removing HTMA and SMBA from solution results from sorption by soils sediments and wastewater treatment solids. The sorption mechanism.
May be harmful if inhaled. Ammonium chloride fume may cause an asthma-like allergy. Future exposure may cause asthma attacks with shortness of breath wheezing coughing andor chest tightness.
Prolonged or repeated skin contact may cause dermatitis. Section 4 - First Aid Measures Eyes. Immediately flush eyes with plenty of water for at least 15 minutes occasionally lifting the.
Boiling point - the temperature at which a liquid turns into a gas. Melting point - the temperature at which a solid turns into a liquid. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.
For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Barium chloride and potassium sulfate are both ionic compounds. We would expect them to undergo a double displacement reaction with each other.
BaCl 2 K 2 SO 4 BaSO 4 2 KCl By examining the solubility rules we see that while most sulfates are soluble barium sulfate is not. Because it is insoluble in water we know that it is the. Urea ammonium sulfate potassium sulfate and potassium chloride.
Complex fertilizers contain two to three primary plant nutrients of which two primary nutrients are in chemical combination. These fertilizers are usually produced in granular form. DAP nitrophosphate and ammonium phosphate.
Ksp solubility product constants of many popular salts at SolubilityOFthings. Cu 3 AsO 4 2. CopperII iodate monohydrate.
The solubility rules are only for ionic solids ability to dissolve in water. While we can calculate the solubility by measuring each substance and following an equation the solubility rules allow us to determine the solubility of a substance before you attempt to create it. It is very important that the rules on this list are followed in order because if a rule seems to.
Illustrations of solubility concepts. Metabolic intermediates lipid bilayer membranes soaps and detergents. Because water is the biological solvent most biological organic molecules in order to maintain water-solubility contain one or more charged functional groups.
These are most often phosphate ammonium or carboxylate all of which are. It has good solubility in water but is very toxic to aquatic life forms. When burned it produces toxic fumes including ammonia chlorine and nitrogen oxide.
It is highly corrosive to the skin eyes and respiratory tract. Benzalkonium chloride is a weak allergen but is highly toxic in large amounts and over time compounding or long-term exposure. Other names for benzalkonium chloride are.
Nitrogen gas does not react with water. It does dissolve in water. Solubility of nitrogen and nitrogen compounds.
Nitrogen N 2 solubility at 20 o C and pressure 1 bar is approximately 20 mgL. Nitrogen solubility may differ between compounds. Nitrogen I oxide solubility is 12 gL and nitriloacetate salt solubility is 640 gL whereas nitrogen chloride is water insoluble.
Ammonium acetate 10 M 771 g NH 4C 2H 3O 2 1761901 M 77 g 7708 Ammonium chloride 10 M 535 g NH 4Cl 05 M 267 g 5349 Ammonium nitrate 10 M CaOH800 g NH 4NO 3 05 M 400 g 8004 01 M 80 g Ammonium sulfate 01 M 132 g NH 4 2SO 4 1321 Barium chloride 01 M 244 g BaCl 2 2H 2O 24428 Barium hydroxide 01 M 315 3g BaOH 2.