
Your Safer Source for Science Supplies Name Formula FW. What is the molar mass of.
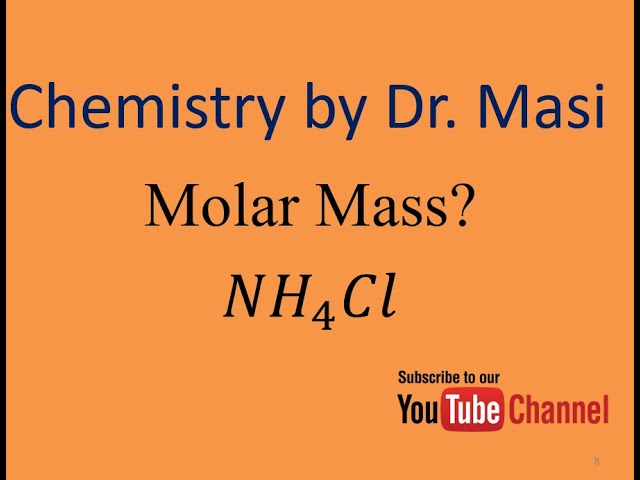
NIOSH Pocket Guide to Chemical Hazards.
Ammonium chloride molar mass. Ammonium chloride is an inorganic compound with the formula NH 4 Cl and a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. In its naturally occurring mineralogic form it is known as sal ammoniacThe mineral is commonly formed on burning coal dumps from condensation of coal-derived gases.
It is also found around some types of volcanic vents. Ammonium Chloride NH 4 Cl- Ammonium chloride is an inorganic compound with formula NH 4 Cl. In its pure form it is white crystalline salt.
Ammonium chloride is used in veterinary medicine in the prevention of urinary stones in sheep goats and cattle. To learn more about the Structure Properties Preparation Uses and FAQs of Ammonium Chloride NH4Cl Visit BYJUS for more. Ammonium Chloride is a systemic and urinary acidifying salt.
Ammonium chloride helps maintain pH and exerts a mild diuretic effect. This acid forming salt also exerts an expectorant effect by irritating the mucous membranes and is used for alleviation of cough. Molar Mass of Frequently Calculated Chemicals.
C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4.
Molar Mass of ammonium chloride NH4Cl Molar Mass of baking powder NaHCO3 Molar Mass of Sucrose C12H22O11 Molar Mass of Isopropyl Alcohol C3H6CHOH Molar Mass of Carbon Monoxide CO Molar Mass of antimony chloride - 3 SbCl3 Molar Mass of Chloroplatinic acid H2PtCl6 Molar Mass of Lactic Acid C3H6O3 Molar Mass of Mercury Sulfate HgSO4 Molar Mass of Potassium. Our molar mass calculator has this for a variety of other compounds. Molar Mass of Ammonium aluminium sulfate AlNH4SO42 Molar Mass of Ammonium nitrate NH4NO3 Molar Mass of Barium sulfate BaSO4 Molar Mass of Calcium carbonate CaCO3 Molar Mass of carbolic acid C6H5OH Molar Mass of graphite C Molar Mass of methane CH4 Molar Mass of hydrogen cyanide.
Ammonium cation is found in a variety of salts such as ammonium carbonate ammonium chloride and ammonium nitrateMost simple ammonium salts are very soluble in water. An exception is ammonium hexachloroplatinate the formation of which was once used as a test for ammoniumThe ammonium salts of nitrate and especially perchlorate are highly explosive in these cases ammonium is the reducing. The molar mass of any substance is the mass in grams of one mole of representative particles of that substance.
The representative particles can be atoms molecules or formula units of ionic compounds. This relationship is frequently used in the laboratory. The simplest type of manipulation using molar mass as a conversion factor is a mole-mass conversion or its reverse a mass-mole.
What is the molar mass of. Make sure to show clearly defined and complete work here 1. Conversion between moles and mass.
How many moles are in 1225 g of magnesium hydroxide. How many moles are in 245 g of hydrogen gas H2. How many grams are in 63 moles of ammonium chloride.
What is the mass of 0288 moles of plumbous. IronIIIChloride AmmoniumSulfide IronIIISulfide AmmoniumChloride Reaction type. Double replacement Please tell about this free chemistry software to your friends.
Direct link to this balanced equation. Instructions on balancing chemical equations. Enter an equation of a chemical reaction and click Balance.
The answer will appear below. Always use the upper case for the first. Chemical Reaction Formula Atomic Mass Formula Chemical Formula Enthalpy Formula Entropy.
Formula Ozone Formula Silane Formula Vanadium pentoxide Formula Stannous Oxide Formula Potassium dichromate Formula Ammonium Chloride Formula Zinc II chloride Formula Ehylene oxide Formula Strontium fluoride Formula Strontium chloride Formula Tungsten IV carbide Formula. The mass of a mole of any element or compound. A concentration unit M.
Defined as the number of moles of solute divided by liters of solution. Your Safer Source for Science Supplies Name Formula FW. Concentration gL Aluminum chloride 02 M 483 g AlCl 3 6H 2O 005 M 121 g 24143 Aluminum nitrate 01 M 375 g AlNO 3 3 9H 2O 37513 Aluminum sulfate.
A 200 g sample of limestone was dissolved in hydrochloric acid and all the calcium present in the sample was converted to Ca 2 aq. Excess ammonium oxalate solution NH 4 2 C 2 O 4aq was added to the solution to precipitate the calcium ions as calcium oxalate CaC 2 O 4s. The precipitate was filtered dried and weighed to a constant mass of 243 g.
The mass of the ammonium chloride is first converted to moles. Then the molarity is calculated by dividing by liters. Note the given volume has been converted to liters.
Think about your result. The molarity is 1579 M meaning that a liter of the solution would contain 1579 mol NH 4 Cl. Four significant figures are appropriate.
In a laboratory situation a chemist must. The molar mass of a compound or an element is the mass per mole of the substance. The value is given in gmole or gramsmole.
You can determine the molecular weight by looking up the numbers under the element names in the periodic table. For example the chlorine molar mass is 3545 gramsmol. Ammonium Ca2 calcium Fe2 ironII Cu2 copperII Mg2 magnesium Ni2 nickelII Sr2 strontium Zn2 zinc Sn2 tinII Pb2 leadII Ba2 barium Fe3 ironIII Al3 aluminium Cr3 ChromiumIII F-fluoride Cl-chloride Br-bromide I-iodide HO-hydroxide NO 2-nitrite NO 3-nitrate HCO 3 -hydrogencarbonate O2-oxide CO 3 2-carbonate SO 4 2-sulfate HSO 4-hydrogensulfate S2-sulphide N3-nitride PO 4 3.
NIST Mass Spectrometry Data Center. 3225 Other Experimental Properties. 1 ppm 149 mgcu m Hydrogen chloride NIOSH.
NIOSH Pocket Guide to Chemical Hazards. Department of Health Human Services Centers for Disease Control Prevention. National Institute for Occupational Safety Health.
DHHS NIOSH Publication No.