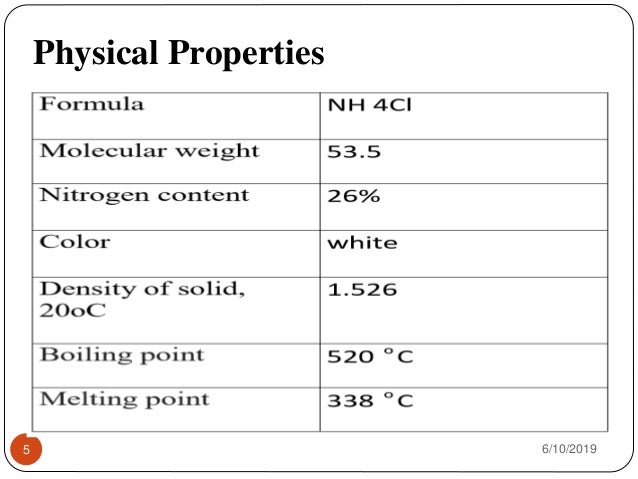
Ammonium chloride is an inorganic compound with the formula NH 4 Cl and a white crystalline salt that is highly soluble in water. Some other physical and chemical properties of potassium chloride are discussed in this subsection.
To view the complete list of companies product names and percent didecyl dimethyl ammonium chloride in formulated products click the following url and enter the CAS Registry number in the Active Ingredient field.
Ammonium chloride chemical properties. The acid-forming properties of ammonium chloride result from dissociation of the salt to an ammonium cation and a chloride anion. In patients with normal hepatic function the ammonium cation is converted to urea by the liver and a hydrogen cation is released which reacts with a bicarbonate ion to form water and carbon dioxide. Ammonium chloride is an inorganic compound with the formula NH 4 Cl and a white crystalline salt that is highly soluble in water.
Solutions of ammonium chloride are mildly acidic. In its naturally occurring mineralogic form it is known as sal ammoniacThe mineral is commonly formed on burning coal dumps from condensation of coal-derived gases. It is also found around some types of volcanic vents.
Ammonium Chloride NH 4 Cl- Ammonium chloride is an inorganic compound with formula NH 4 Cl. In its pure form it is white crystalline salt. Ammonium chloride is used in veterinary medicine in the prevention of urinary stones in sheep goats and cattle.
To learn more about the Structure Properties Preparation Uses and FAQs of Ammonium Chloride NH4Cl Visit BYJUS for more. The ammonium cation is a positively charged polyatomic ion with the chemical formula NH 4. It is formed by the protonation of ammonia NH 3.
Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations NR 4 where one or more hydrogen atoms are replaced by organic groups indicated by R. The National Pesticide Information Retrieval System NPIRS identifies 74 companies with active labels for products containing the chemical didecyl dimethyl ammonium chloride. To view the complete list of companies product names and percent didecyl dimethyl ammonium chloride in formulated products click the following url and enter the CAS Registry number in the Active Ingredient field.
Ammonium Chloride Safety Data Sheet according to Federal Register Vol. 58 Monday March 26 2012 Rules and Regulations Issue date. 22 05212020 EN English US Page 1 SECTION 1.
Substance Substance name. 12125-02-9 Product code. Quaternary ammonium compound l-carnitine 3-hydroxy-4-N-trimethylammonio-butanoate has been shown to possess free-radical scavenging and antioxidant properties and was found to ameliorate oxidative organ injury in various pathologic conditions such as coronary heart disease heart or renal failure and ischemia-reperfusion 3848.
Carnitine can be synthesized from the amino acids lysine and. 10 mgm3 TWA Personal Protective Equipment Eyes. Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHAs eye and face protection regulations in 29 CFR 1910133 or European Standard EN166.
Wear appropriate protective gloves to prevent skin exposure. 12125-02-9 Ammonium Chloride ACGIH TLV. 10mgm3 12125-02-9 Ammonium Chloride TWA 10 mgm3 USA.
NIOSH 12125-02-9 Ammonium Chloride TWA 10 mgm3 USA. Safety Data Sheet according to 29CFR19101200 and GHS Rev. 3 Effective date.
01062015 Page 4 of 7 Ammonium ChlorideLab Grade Created by Global Safety Management Inc. Benzalkonium chlorides BACs are chemicals with widespread applications due to their broad-spectrum antimicrobial properties against bacteria fungi and viruses. This review provides an overview of the market for BACs as well as regulatory measures.
Quaternary ammonium compounds quats such as benzalkonium chloride see Fig. 134 for the chemical structure are a large group of related compounds. Some concentrated formulations have been shown to be effective low-level disinfectants.
Typically quats do not exhibit efficacy against difficult-to-kill nonenveloped viruses such as norovirus rotavirus or poliovirus. Some other physical and chemical properties of potassium chloride are discussed in this subsection. The crystals of potassium chloride are made up of face-centred cubic FCC unit cells.
The molar mass of KCl is 745513 gramsmol. Its density in the solid crystalline form is 1984 grams per cubic centimetre. The melting and boiling points of potassium chloride are 1040 K.
Corrosion - Corrosion in piping systems - caused by thermodynamic and electrochemical processes - corrosion problems and methods of protection and prevention. Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. Chemical Resistance of Rubbers and Elastomers - Rsistance to chemicals.