Appendix A Hazardous Materials Table. NH 4 NO 3.

Ammonium cation is found in a variety of salts such as ammonium carbonate ammonium chloride and ammonium nitrateMost simple ammonium salts are very soluble in water.
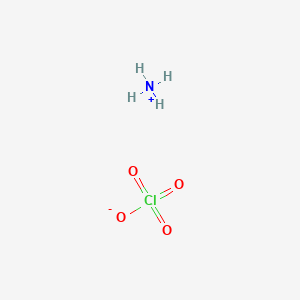
Ammonium chlorate mass. Ammonium perchlorate AP is an inorganic compound with the formula NH 4 ClO 4. It is a colorless or white solid that is soluble in water. It is a powerful oxidizer.
Combined with a fuel it can be used as a rocket propellant. Its instability has involved it in a number of accidents such as the PEPCON disaster. Ammonium cation is found in a variety of salts such as ammonium carbonate ammonium chloride and ammonium nitrateMost simple ammonium salts are very soluble in water.
An exception is ammonium hexachloroplatinate the formation of which was once used as a test for ammoniumThe ammonium salts of nitrate and especially perchlorate are highly explosive in these cases ammonium is the reducing. A mixture of potassium chlorate and sodium amide explodes Mellor 8258. If a drop of a solution of sulfur dioxide in ether or alcohol is added to powdered potassium chlorate the mass explodes Mellor 2311.
Potassium chlorate and sulfuric acid react to cause fire and possible explosions Mellor 2315. CID 222 Ammonia CID 123351 Perchlorate CID 24247 Perchloric acid Dates. Ammonium perchlorate appears as a white crystalline solid or powder.
Classified as a division 11 explosive if powdered into particles smaller than 15 microns in diameter or if powdered into larger. Molar Mass of Frequently Calculated Chemicals. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver.
Sodium chlorateV NaClO3 contains 216 by mass of sodium 333 by mass of chlorine and 451 by mass of oxygen. A Use the above data to show that the. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. RESULTS AND DISCUSSIONS A. Similarly the molar mass of Ammonium Sulfide is 66.
For a solution mass percent equals the mass of an element in one mole of the compound divided by the molar mass of the compound multiplied by 100. FeCO 3 Iron II Carbonate insoluble 4. 4 aq Ammonium sulfate 2 NH 4 -aq SO 4 2aq 3.
All nitrates acetates and. Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more. Modification of work by vxlaFlickr.
Modification of work by the Italian voiceFlickr. Modification of work. Sodium chlorate was mixed with the energetic fuel nitrobenzene to make small explosive charges.
To counter the threat of chlorate explosives the United Kingdom government mandated the addition of a diluent to weed killer to reduce its explosive potential. After chlorate was no longer an option PIRA turned to AN. Many farmers in Northern Ireland possessed large quantities of AN as it was a.
Ammonium Fe CN 6 4. Mg2 magnesium CO 3 2 carbonate ClO 3 chlorate Ca2 calcium HCO 3 hydrogen carbonate ClO 4 perchlorate Sr2 strontium SO 3 2 sulfite IO 3 iodate Ba2 barium HSO 3 hydrogen sulfite MnO 4 permanganate Al3 aluminum SO 4 2 sulfate NO 2 nitrite Sn2 tinII HSO 4 hydrogen sulfate NO 3 nitrate Sn4 tinIV S 2O 3 2 thiosulfate OH. U atomic mass unit atomic mass C.
Ions That Form Soluble Compounds Exceptions Group 1 ions Li Na etc ammonium NH 4 nitrate NO 3 acetate C 2 H 3 O 2 or CH 3 COO hydrogen carbonate HCO 3 chlorate ClO 3 halides Cl Br I when combined with Ag Pb2 or Hg 2 2 sulfates SO 4 2 when combined with Ag Ca2 Sr2 Ba2 or Pb2 Ions That Form. Nitrate and ammonium are the. Chlorate-sensitive CSSSL NI10-1 was identified from a BC 4 F 4 population containing a single fragment of chromosome 10 from IR24.
A NI10-1 Nipponbare F 1. Chemical formula plays an important role in understanding different concepts of chemistry. Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand.
Which of the following salt can be used to differentiate between sodium hydroxide and ammonium hydroxide solution fecl2 cano32 fecl3 mgno32 Iupac name What is the iupac name of this compound a gas cylinder of capacity 100 litres is filled with a gas X whose mass is 50 gram when the same cylinder is filled with hydrogen gas at the same temp. Erature and pressure the mass of. CH 3 COONH 4.
Ammonium carbonate NH 4 2 CO 3. Ammonium dichromate NH 4 2 Cr 2 O 7. NH 4 NO 3.
Ammonium oxalate NH 4 2 C 2 O 4. A reaction in which a reducing agent loses electrons while it is oxidized and the oxidizing agent gains electrons while it is reduced is called as redox oxidation reduction reaction. An unbalanced redox reaction can be balanced using this calculator.
Percent composition in chemistry typically refers to the percent each element is of the compounds total mass. The basic equation mass of element mass of compound X 100. For instance if you had a 800 g sample of a compound that was 200 g element X and 600 g element y then the percent composition of each element would be.
Appendix A Hazardous Materials Table. Postal Service Mailability Guide. The mailing information in this table is based on the online DOT Hazardous Materials Table in.
Group VA Group IVA. PO 4 3-phosphate. AsO 4 3-arsenate.
SiO 3 2-silicate But note that nitrogen does not follow this pattern ie nitrate NO 3- Some nonmetals form a series of polyatomic ions with oxygen all having the same charge. The oxidizer is usually ammonium nitrate potassium chlorate or ammonium chlorate and often comprises as much as four-fifths or more of the whole propellant mix. The fuels used are hydrocarbons such as asphaltic-type compounds or plastics.
Because the oxidizer has no significant structural strength the fuel must not only perform well but must also supply the necessary form and rigidity to. 1 Ba 2 ion is required to balance 2 OH- ions Ammonium has a 1 charge and phosphate has a -3 charge therefore 3 NH 4 ions are required to balance 1 PO 4 3- ion Potassium has a 1 charge and sulfate has a -2 charge therefore 2 K ions are required to balance 1 SO 4 2- ion. NH 4 3 PO 4 3.
K 2 SO 4. In association with Nuffield Foundation. Try this demonstration to create a mini volcanic eruption illustrating the decomposition of ammonium dichromate.
Includes kit list and safety instructions. In association with Nuffield Foundation. Explore an application of electrolysis in this demonstration by anodising aluminium to improve corrosion.
How do you know the Order of Elements in a Chemical Formula What is Chemical Formula. A chemical formula is a representation of a chemical substance using letters for atoms and subscript numbers to show the numbers of each type of atoms that are present in the substance. Based on the chemical formula of a substance we know the composition of the substance.
Calculations of empirical formula may involve composition by mass or percentage composition by mass data. Be able to write balanced full and ionic equations including state symbols for chemical reactions. Be able to calculate amounts of substances in mol in reactions involving mass volume of gas volume of solution and concentration.