Write each chemical formula and name each compound correctly. 3 Chlorate ion.

78 ammonium oxide NH 4 2 O 79 tin IV selenide SnSe 2 80 carbon tetrachloride CCl 4 81 Which of the following pairs of elements would most likely form a ionic compound.
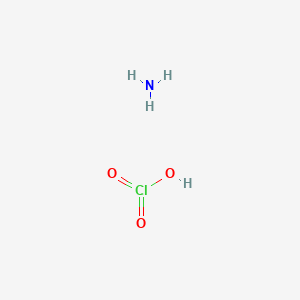
Ammonium chlorate compound formula. Ammonium perchlorate AP is an inorganic compound with the formula NH 4 ClO 4. It is a colorless or white solid that is soluble in water. It is a powerful oxidizer.
Combined with a fuel it can be used as a rocket propellant. Its instability has involved it in a number of accidents such as the PEPCON disaster. Ionic Compound Naming and Formula Writing List 1.
Copy this to my account. E-mail to a friend. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A.
Ammonium perchlorate an oxidizer is the most prevalent form of this compound. Has been widely used in solid propellants fireworks and flares. And is a constituent of many munition components.
Manufacture of ammonium perchlorate began in the 1940s primarily for use by the defense industry and later by the aerospace industry. The ammonium cation is a positively charged polyatomic ion with the chemical formula NH 4. It is formed by the protonation of ammonia NH 3.
Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations NR 4 where one or more hydrogen atoms are replaced by organic groups indicated by R. Polyatomic ions are ions which consist of more than one atom. For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atomsThe atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit.
A mixture of potassium chlorate and sodium amide explodes Mellor 8258. If a drop of a solution of sulfur dioxide in ether or alcohol is added to powdered potassium chlorate the mass explodes Mellor 2311. Potassium chlorate and sulfuric acid react to cause fire and possible explosions Mellor 2315.
Sulfuric acid 98072 g. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. The chemical formula of a compound is a symbolic representation of its chemical composition.
Chemical formulae provide insight into the elements that constitute the molecules of a compound and also the ratio in which the atoms of these elements combine to form such molecules. For example the chemical formula of water which is H 2 O suggests that two hydrogen atoms combine with one oxygen. 78 ammonium oxide NH 4 2 O 79 tin IV selenide SnSe 2 80 carbon tetrachloride CCl 4 81 Which of the following pairs of elements would most likely form a ionic compound.
A Ca and Ni both metals B Cu and Ar Noble gases normally do not bond C F and. 4 Ammonium ion F Fluoride ion. 3 Chlorate ion.
Most Common Formula Representations All represent ethanol Example C 2H 6O CH 3CH 2OH Name Molecular Formula Condensed Molecular Formula Structural Formula Line Formula. Microsoft Word - Chem 1 Compound Sheet FS08 Author. Terry Bone Created Date.
922008 15513 PM. Ionic Naming Practice Problems - Solutions Name the following ionic compounds. 1 NaBr sodium bromide 2 ScOH3 scandium hydroxide 3 V2SO43 vanadium III sulfate 4 NH4F ammonium fluoride 5 CaCO3 calcium carbonate 6 NiPO4 nickel III phosphate 7 Li2SO3 lithium sulfite 8 Zn3P2 zinc phosphide 9 SrC2H3O22 trontium acetates.
30 copper II chlorate CuClO 3 2 31 cobalt III chromate Co 2 CrO 4 3 32 ammonium oxide NH 4 2 O 33 potassium hydroxide KOH 34 lead IV sulfate PbSO 4 2 35 silver cyanide AgCN 36 vanadium V nitride V 3 N 5 37 strontium acetate SrC 2 H 3 O 2 2 38 molybdenum sulfate MoSO 4 3 39 platinum II sulfide PtS 40 ammonium. FORMULAS AND NOMENCLATURE OF IONIC AND COVALENT COMPOUNDS Adapted from McMurryFay section 210 p. 56 -63 and the 1411 Lab Manual p.
TYPES OF COMPOUNDS Ionic compounds are compounds composed of ions charged particles that form. Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more. Modification of work by vxlaFlickr.
Modification of work by the Italian voiceFlickr. Modification of work. I Calcium chlorate H 2 SO 4 A Sulfuric Acid SF 6 M Sulfur hexafluoride Identify whether the compounds is an Ionic Molecular or Acidic Compound.
Then write the formula. Formula Type Chemical Name N 2 H 6 M Dinitrogen hexahydride HNO 2 A Nitrous Acid CO M Carbon monoxide HCl A Hydrochloric Acid S 4 Cl 8 M Tetrasulfur octachloride CaOH 2 I Cadmium hydroxide H 2 CrO 4 A Chromic acid MgSO 4 I. But note that nitrogen does not follow this pattern ie nitrate NO 3-.
Some nonmetals form a series of polyatomic ions with oxygen all having the same charge. The -ate forms formula and charge must be memorizedIn some cases the -ate form has three oxygens and in some cases four oxygens. Write each chemical formula and name each compound correctly.
The instructor should also review the score for forming each compound. Scoring is described in step 5 of the procedure. This activity is a game but it is also meant to be a teaching tool for proper naming procedures.
Students will hopefully learn by repeatedly practicing naming simple compounds. Instructors should emphasize team. Writing the formula of a compound.
Write the symbols of formulae of the ions of the compound side by side with positive ion on the left hand side and negative ion on right hand side. Enclose the polyatomic ion in a bracket. Write the valency of each ion below its symbol Step-4.
Reduce the valency numerals to a simple ratio by dividing with a common factor if any. Percent composition in chemistry typically refers to the percent each element is of the compounds total mass. The basic equation mass of element mass of compound X 100.
For instance if you had a 800 g sample of a compound that was 200 g element X and 600 g element y then the percent composition of each element would be. The resulting compound carries a neutral electrical charge. For example table salt or sodium chloride consists of the Na cation bonded to the Cl-anion to form NaCl.
Salts are hygroscopic or tend to pick up water. This water is called water of hydration. By convention the cation name and formula are listed before the anion name and formula.
In other words write the cation on the left. Saturated Definition 1. This chemistry definition refers to a saturated compoundA saturated substance is one in which the atoms are linked by single bondsA fully saturated compound contains no double or triple bonds.
Alternatively if a molecule contains double or triple bonds it is considered to be unsaturated. When a compound contains a polyatomic ion special rules apply to writing the compound formula. For example calcium nitrate contains two nitrate ions.
Its formula is CaNO₃₂. The name of the. The notation of hydrous compound nH 2 O where n is the number of water molecules per formula unit of the salt is commonly used to show that a salt is hydrated.
The latexcdotlatex indicates that the water is loosely bonded to the ionic compound. The n is usually a low integer though it is possible for fractional values to exist. The prefixes are the same Greek prefixes.
We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production.