These solutions are named by adding the prefix hydro- to the name of the compound and then replacing the suffix -ide with -icFor example hydrogen chloride HCl dissolves in water to form hydrochloric acid. These solutions are named by adding the prefix hydro- to the name of the compound and then replacing the suffix -ide with -icFor example hydrogen chloride HCl dissolves in water to form hydrochloric acid.

When dilute sulphuric acid reacts with the metal zinc zinc sulphate is formed with the evolution of hydrogen gas.
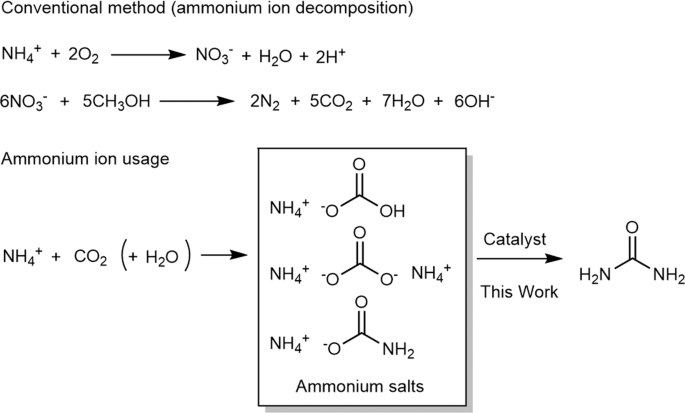
Ammonium bicarbonate reacts with acids. Ammonium bicarbonate is an inorganic compound with formula NH 4HCO 3 simplified to NH 5 CO 3. The compound has many names reflecting its long history. Chemically speaking it is the bicarbonate salt of the ammonium ion.
It is a colourless solid that degrades readily to carbon dioxide water and ammonia. Ammonium bicarbonate is produced by combining carbon dioxide and ammonia. The ammonium ion is generated when ammonia a weak base reacts with Brønsted acids proton donors.
H NH 3 NH 4. The ammonium ion is mildly acidic reacting with Brønsted bases to return to the uncharged ammonia molecule. NH 4 B HB NH 3.
Thus treatment of concentrated solutions of ammonium salts with strong base gives ammonia. When ammonia is dissolved in water a. Weak Acids dissociate partially in water and are efficient at preventing pH changes.
It reacts with the carbonic acid to form sodium bicarbonate a weak base The pH of the solution rises only slightly. This system is the only important ECF buffer. Nearly identical to the bicarbonate system.
Sodium salts of dihydrogen phosphate NaH 2 PO. Quaternary ammonium compounds quats. A typical proton scavenger is potassium bicarbonate.
This is often used with a polar solvent like methanol that is able to solubilize a portion of the inorganic salt. The reaction of potassium bicarbonate with the proton yields water carbon dioxide and a potassium halide. After the reaction is complete monitored by thin layer chromatography or NMR.
Hydrochloric acid reacts with sodium hydroxide to produce sodium chloride common salt water and heat. The reaction is represented by the following equation. HCl NaOH NaCl H 2 O 2.
Reaction of metals with acids - Metals react with acids to replace hydrogen from the acids thereby producing salts and liberating hydrogen gas. Acids react with metals to produce salt by displacing hydrogen. When dilute sulphuric acid reacts with the metal zinc zinc sulphate is formed with the evolution of hydrogen gas.
Zn H 2 SO 4 ZnSO 4 H 2 ii. Zinc is the only metal which reacts with sodium hydroxide to form sodium zincate with the release of hydrogen gas. Zn 2NaOH Na 2 ZnO 2 H 2.
This substance is readily absorbed by the gastrointestinal tract and utilized in the liver to form amino acids and proteins. When ammonium ions are converted to urea liberated hydrogen ion reacts with bicarbonate ion to form water and carbon dioxide. The chloride ion displaces the bicarbonate ion.
Chloride is loaded into the kidneys. The increased chloride concentration in the extracellular. Reactions between acids and carbonates.
This page looks at the reactions between acids and carbonates to give a salt carbon dioxide and water. Acid carbonate salt CO 2 water. Reactions involving calcium carbonate.
The commonest carbonate-acid reaction you will come across is that between calcium carbonate and dilute hydrochloric acid. Calcium carbonate occurs. In this case the value of K b for bicarbonate is greater than the value of K a for ammonium.
Therefore bicarbonate is a slightly more alkaline than ammonium is acidic and a solution of ammonium bicarbonate in pure water will be slightly basic pH 70. In summary when a salt contains two ions that hydrolyze compare their K a and K b values. Simple covalent compounds that contain hydrogen such as HCl HBr and HCN often dissolve in water to produce acids.
These solutions are named by adding the prefix hydro- to the name of the compound and then replacing the suffix -ide with -icFor example hydrogen chloride HCl dissolves in water to form hydrochloric acid. Hydrogen bromide HBr forms hydrobromic acid. Acids are sour in taste turn blue litmus red and dissolve in water to release H ions.
Sulphuric acid H 2 SO 4 Acetic Acid CH 3 COOH Nitric Acid HNO 3 etc. Acids have a sour taste. Turns blue litmus red.
Acid solution conducts electricity. Release H ions in aqueous solution. Acids are divided into two types on the basis of.
Acids reacts with metal to produce hydrogen gas thus it spoils the food and also forms some toxic metal compounds which makes the food stuffs to become poisonous. A What is produced if an acid is added to a base. B Why does dry HCl gas not change the colour of dry litmus paper.
C What colour does phenolphthalein indicator turn when added to an alkali such as sodium hydroxide. The kidneys have the predominant role in regulating the systemic bicarbonate concentration and hence the metabolic component of acid-base balance. This function of the kidneys has two components.
Reabsorption of virtually all of the filtered HCO 3 and production of new bicarbonate to replace that consumed by normal or pathologic acids. This production or generation of. It is an oxidizing reagent it readily reacts with reducing materials in large quantity it may produce a violent reaction.
Direct exposure to heat or shock will explode it. When heated to decomposition it emits toxic fumes of ammonia and nitrogen oxides Sax 9th ed 1996 p. Acids Bases and Salts Class 10 MCQs Questions with Answers.
Solving the Acids Bases and Salts Multiple Choice Questions of Class 10 Science Chapter 2 MCQ can be of extreme help as you will be aware of all the concepts. These MCQ Questions on Acids Bases and Salts Class 10 with answers pave for a quick revision of the Chapter thereby helping you to enhance subject knowledge. Acids and bases exist as conjugate acid-base pairs.
When a strong acid dissolves in water the acid reacts extensively with water to form H 3 O and A-ions. Only a small residual concentration of the HA molecules remains in solution The product of the concentrations of the H 3 O and A-ions is therefore much larger than the concentration of the HA molecules so K a for a strong acid. AAmmonium NH 4 Nitrate NO 3-Hydride H-Chloride Cl-Ammonium is odd because Ammonium is cation and rest are anions.
BHydrogen chloride is odd because Hydrogen chloride is acid and rest are base. CAcetic acid CH 3 COOH Carbonic acid H 2 CO 3 Hydrochloric acid HCl Nitric acid - HNO 3 HCl is the only Diatomic Hetro-Nuclear compound and remaining are Poly Atomic compound. The older Arrhenius theory of acids and bases viewed them as substances which produce hydrogen ions or hydroxide ions on dissociation.
As useful a concept as this has been it was unable to explain why NH 3 which contains no OH ions is a base and not an acid why a solution of FeCl 3 is acidic or why a solution of Na 2 S is alkaline. A more general theory of acids and bases was. In these reactions the carboxylic acids act like inorganic acids.
They neutralize basic compounds. With solutions of carbonate CO 3 and bicarbonate HCO 3 ions they also form carbon dioxide gas. Carboxylic acid salts are named in the same manner as inorganic salts.
The name of the cation is followed by the name of the organic anion. Ammonium preferably with ventilation corrosive cabinet or storage area Calcium potential water sources Chemical Segregation and Storage Table Chemical Segregation Class of Chemicals Common Chemical Examples Additional Concerns and Storage Recommendations Common Incompatible Chemical Types Possible Reaction if MixedHealth Concerns Corrosive Acids-Organic Acid Glacial Acetic Acid Butyric. Urea is naturally produced when the liver breaks down protein or amino acids and ammonia.
The kidneys then transfer the urea from the blood to the urine. Extra nitrogen is expelled from the body through urea and because it is extremely soluble it is a very efficient process. The average person excretes about 30 grams of urea a day mostly through urine but a small amount is also secreted.
It reacts with other ingredients to release carbon dioxide which helps the dough rise. A diluted solution of household baking soda can treat heartburn and indigestion. It functions as a mouthwash treats gum diseases and relieves insect bites.
A hydrogen peroxide and sodium bicarbonate paste can be used as an alternative to commercial toothpaste. Baking soda is an effective cleaning. Mcq on acids bases and salts class 10 pdf class 10 science chapter 2 mcq online test mcq on acids bases and salts class 10 icse acid bases and salts class 10 mcq online test 100 mcq from acid bases and salts class 10 quiz on acids bases and salts for class 10 questions on acids bases and salts class 10 multiple choice questions on acids bases and salts for class 10.
Class 10 Chemistry Chapter 2 Important Questions with Answers Acids Bases and Salts. Acids Bases and Salts Class 10 Important Questions Very Short Answer Type. How is the concentration of hydronium H 3 O ions affected when a solution of an acid is diluted.
When an acid solution is diluted with water then concentration of H 3 O ions gets decreased. Water can also act as an acid as when it reacts with ammonia. The equation given for this reaction is.
H 2 O NH 3 OH NH 4 Here H 2 O donates a proton to NH 3. The hydroxide ion is the conjugate base of water which acts as an acid and the ammonium ion is the conjugate acid of the base ammonia.