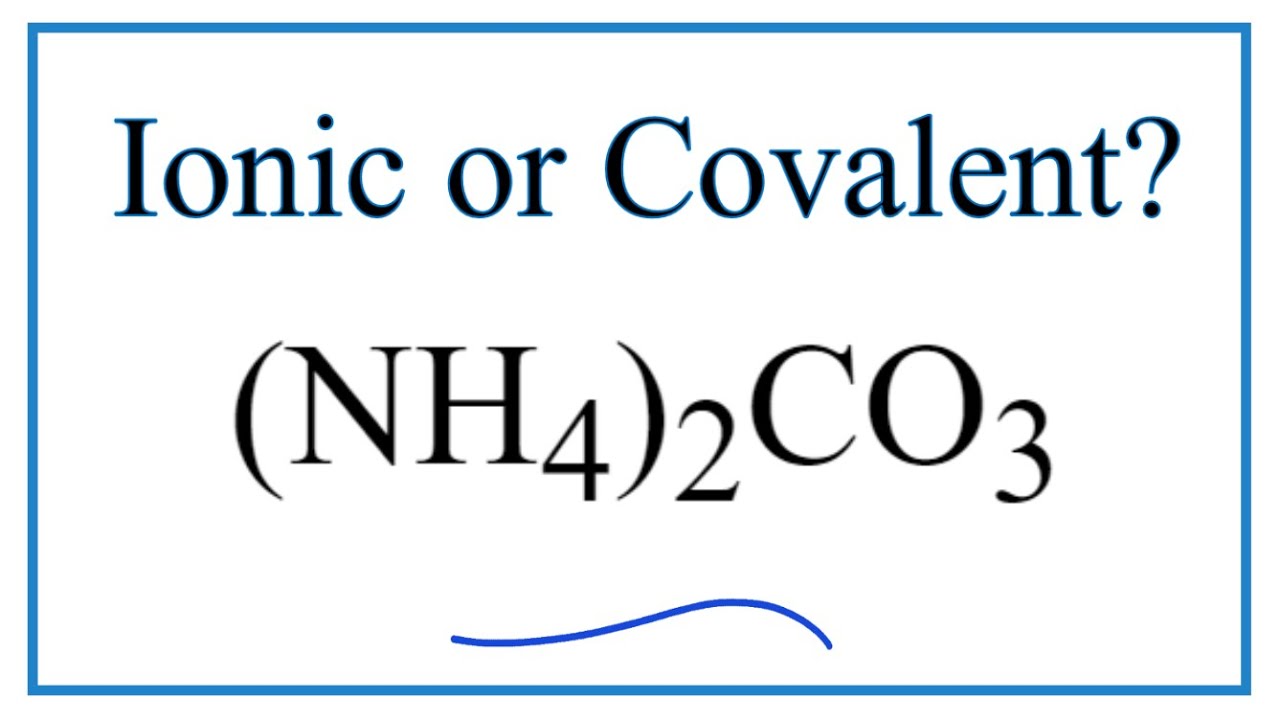
Monatomic positive ions have the name of the element from which they are formed. Carbon monoxide - CO.
Modification of work.
Ammonium bicarbonate formula. Ammonium bicarbonate is an inorganic compound with formula NH 4HCO 3 simplified to NH 5 CO 3The compound has many names reflecting its long history. Chemically speaking it is the bicarbonate salt of the ammonium ion. It is a colourless solid that degrades readily to.
Ammonium bicarbonate is manufactured by passing carbon dioxide gas countercurrently through a descending stream of aqua ammonia. The reaction is normally carried out in a packed tower or absorption column. Because the reaction is exothermic cooling the lower portion of the tower is advisable.
Concentration of the solution is monitored by. The ammonium cation is a positively charged polyatomic ion with the chemical formula NH 4. It is formed by the protonation of ammonia NH 3.
Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations NR 4 where one or more hydrogen atoms are replaced by organic groups indicated by R. Sodium bicarbonate should be used with extreme caution in patients with congestive heart failure or other edematous or sodium-retaining conditions. In patients with renal insufficiency especially those with severe insufficiency such as oliguria or anuria.
And in patients receiving corticosteroids or corticotropin since each gram of sodium bicarbonate contains about 12 mEq of sodium. Polyatomic ions are ions which consist of more than one atom. For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atomsThe atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit.
Sodium bicarbonate is a salt that breaks down to form sodium and bicarbonate in water. This breakdown makes a solution alkaline meaning it is able to neutralize acid. Because of this sodium.
NH 4 HCO 3. Ammonium carbonate formula NH 4 2 CO 3. NH 4 NO 3.
Al 2 S 3. NH 4 NO 2. Chemical formula that corresponds to the name Name Formula 1 NaF 13 potassium fluoride 2 K2CO 3 14 ammonium sulfate 3 MgCl 2 15 magnesium iodide 4 BeOH 2 16 copper II sulfite 5 SrS 17 aluminum phosphate 6 Cu 2S 18 lead II nitrite 7 ZnI 2 19 cobalt II selenide 8 Ca 3PO 42 20 silver cyanide 9 NH 4I 21 copper II bicarbonate 10 MnNO 33 22 iron II oxide 11 FePO.
The first step in making the nitrogen in urea CH4N2O available to plants is by converting it to either ammonia NH3 or ammonium ions NH4 and bicarbonate ions HCO3. Naturally-occurring soil bacteriabroadly-called ammonia-oxidizing bacteria AOBaccomplish this. Chemical formula plays an important role in understanding different concepts of chemistry.
Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand. A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another. Chemical formulas can be.
Ammonium nitrate lime 2126 N. This granular fertiliser is a mixture of ammonium nitrate and lime. It is sold under various trade names.
Because of the calcium carbonate present it does not cause acidity when added to the soil. Urea 46 N. This is the most concentrated solid nitrogen fertiliser and it is marketed in the prilled form.
It is sometimes used for aerial top-dressing. NH42SO4 ammonium sulfate 20. NiS nickel II sulfide Write the chemical formula for each of the following compounds.
Sodium nitrite NaNO2 31. Potassium carbonate K2CO3 22. Iron III oxide Fe2O3 32.
Silver sulfide Ag2S 23. Aluminum hydroxide AlOH3 33. Nickel II carbonate NiCO3 24.
Ammonium hydroxide NH4OH 34. Calcium phosphate Ca3PO42 25. Magnesium chloride MgCl2 35.
The University of Georgia Agricultural and Environmental Services Laboratories offer soil salinity testing to help farmers and the general public diagnose and manage problems associated with soil salinity. By definition a saline soil contains excess soluble salts that reduce the growth of most crops or ornamental plants. This publication discusses soil salinity testing data interpretation.
Formula writing rules to write the correct chemical formulas for each compound. Compound Name Type of Compound. Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate 10 manganese III chloride Compound.
Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more. Modification of work by vxlaFlickr. Modification of work by the Italian voiceFlickr.
Modification of work. Section B Write the formula of the ionic compounds containing polyatomic ions 1. Lead II chlorate PbClO32 2.
Strontium acetate SrC2H3O22 3. Zinc phosphate Zn3PO42 4. Ammonium cyanide NH4CN 5.
Sodium carbonate Na2CO3 6. Lead IV dichromate PbCr2O72 7. Copper I sulfite Cu2SO3 8.
Cadmium phosphate Cd3PO42 9. Tin II bicarbonate. The reducing solution was then removed and a 55-mM iodoacetamide in 100 mM ammonium bicarbonate alkylating solution was added.
Samples were incubated at room temperature for 1 h in the dark. The alkylating solution was then removed and 100 mM ammonium bicarbonate was added. After 5 min an equal volume of acetonitrile was added to make a 11 vv solution.
After 15 min of incubation. 1 ammonium chloride 2 iron III nitrate 3 titanium III bromide 4 copper I phosphide 5 tin IV selenide 6 gallium arsenide 7 lead IV sulfate 8 beryllium bicarbonate 9 manganese III sulfite 10 aluminum cyanide 11 CrPO 4 2 12 VCO 3 2 13 SnNO 2 2 14 Co 2 O 3 15 TiC 2 H 3 O 2 2 16 V 2 S 5 17 CrOH 3 18 LiI 19 Pb. We therefore need a series of rules that allow us to unambiguously name positive and negative ions before we can name the salts these ions form.
Monatomic positive ions have the name of the element from which they are formed. Na sodium. Zn 2 zinc.
Ca 2 calcium. H hydrogen. K potassium.
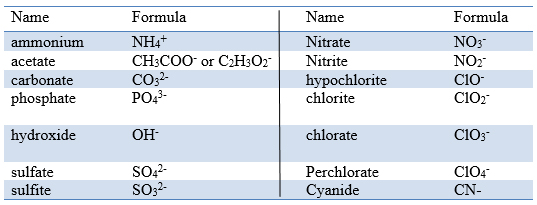
Sr 2 strontium. Name Formula Charge Name Formula Charge Name Formula Charge aluminum Al 3 3 magnesium Mg 2 2 carbonate CO 3 2 2 ammonium NH 4 1 manganese II Mn 2 2 chlorate ClO 3 1 barium Ba 2 2 manganese III Mn 3 3 chloride Cl 1 cadmium Cd 2 2 mercury I mercurous See note Hg 2 2 2 X 1 chromate CrO 4 2 2 calcium Ca 2 2 mercury II mercuric Hg 2 2. Ammonium CO 3 2 carbonate H 3O.
The subscripts in the formula must produce an electrically neutral formula unit. That is the total positive charge must equal the total nega tive charge 3. The subscripts should be the smallest set of whole numbers possible.
If there is only one of a polyatomic ion in the formula do not place parentheses around it. Eg NaNO 3 not NaNO 3. Ammonium bicarbonate - NH 4 HCO 3.
Ammonium chloride - NH 4 Cl. Ammonium hydroxide - NH 4 OH. Calcium nitrate - CaNO 3 2.
Calcium oxide - CaO. Carbon monoxide - CO. Chlorine gas - Cl 2.
Phenol - C 6 H 6 O. You may know HCO3- as bicarbonate. This way of naming uses the prefix bi to indicate the addition of hydrogen bi here has nothing at all to do with two.
The use of bi is archaic but still quite common and you should be familiar with it. What do you think is the formula for bisulfate. Finally in some ions sulfur replaces one of the oxygen atoms and then thio is added as a prefix.
The anion gap increases whenever bicarbonate is lost due to it combining with a hydrogen ion that was previously attached to a conjugate base. When bicarbonate combines with a hydrogen ion the result is carbonic acid H2CO3. The conjugate base can be any negatively charged ion that isnt a bicarbonate or a chloride.
The formula for anion. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production.