
Azelaic acid Dimerized vegetable oil acids Amines. Nitrous acid is also highly volatile in the gas phase it exists predominantly as a trans-planar moleculeIn solution it is unstable with respect to the disproportionation reaction.
At levels not to exceed 02 by weight of lubricants or release agents applied at levels not to exceed 1 lb.
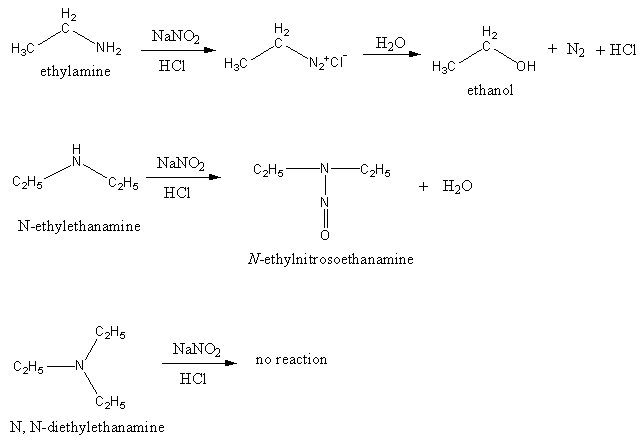
Amine reaction with sodium nitrite. Sodium nitrite is sold as the salt and in solution. The finely crystalline slightly yellowish salt is marketed in untreated form and also after treatment with aryl alkyl sulfonates. The salt contains ca.
990 sodium nitrite 06 sodium nitrate 01 sodium chloride and sodium sulfate and 01 water. An elimination reaction complementary to the Hofmann elimination occurs when 3º-amine oxides are heated at temperatures of 150 to 200 ºC. This reaction is known as the Cope Elimination.
It is commonly carried out by dropwise addition of an amine oxide solution to a heated tube packed with small glass beads. A stream of nitrogen gas flowing. Nitrite is the conjugate base of the weak acid nitrous acid.
HNO 2 H NO 2. PK a 33 at 18 C. Nitrous acid is also highly volatile in the gas phase it exists predominantly as a trans-planar moleculeIn solution it is unstable with respect to the disproportionation reaction.
3HNO 2 aq H 3 O NO 3 2NO. This reaction is slow at 0 C. Sodium nitroprusside is similarly used as a presumptive test for the presence of alkaloids amine-containing natural products common in illicit substances.
The test called Simons test is performed by adding 1 volume of a solution of sodium nitroprusside and acetaldehyde in deionized water to a suspected drug followed by the addition of 2 volumes of an aqueous sodium carbonate solution. Le nitrite de sodium de formule Na N O 2 est le nitrite le plus important dans lindustrie chimique. Son code est E250.
Lancienne méthode de fabrication était basée sur la réduction du nitrate de sodium par le plomb métallique à 420 C. Le nitrite de sodium sobtient de nos jours comme sous-produit lors de la synthèse industrielle de lacide nitrique. Le mélange de.
Amino acids possess an amine group a carboxylic acid group and a varying side chain that differs between different amino acids. There are 20 naturally occurring amino acids which vary from one another with respect to their side chains. Their melting points are extremely high usually exceeding 200C and at their pI they exist as zwitterions rather than as unionized molecules.
The reagent strip test for nitrite is based on the. Use of nitrite by bacteria present in the urine B. Reaction of nitrite with the cell wall of gram-negative bacteria C.
Reduction of nitrate in urine to nitrite by bacteria D. Reaction of bacterial nitrite with an aromatic amine to produce a pH change. A Reaction with nitrous acid Dissolve the amine 05 mL in concentrated acid 20 mL and water 3 mL and cool the solution to 0 - 5 in an ice-bath for 5 minutes.
Add a cold solution ice-bath of sodium nitrite 05 g in water 20 mL from a dropper with swirling of. Signalons que le sodium se dissout dans léthylamine liquide pour donner des paires ions-électrons solvatés comme dans lammoniac liquide. Formation de complexes Les amines sont des bases de Lewis et à ce titre forment de nombreux complexes avec les ions métalliques des éléments de transition.
Les amines simples sont des ligands comparables à lammoniac. Neither the precise chemical nature of the nitrosating agent nor the amine precursor for NDMA in bacon is known with certainty. However the evidence suggests that the nitrosating agent is a reaction product of nitrite and lipids in the bacon.
Dried foods and ingredients. A variety of processes and equipment are used to dry foods and food ingredients. During the direct-fire process air used.
We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Here we report another reaction to add to the click reaction family. The formation of azides from primary amines one of the most abundant functional groups5.
Our purpose is to solve the toughest problems in life science by collaborating with the global scientific community and through that we aim to accelerate access to better health for people everywhere. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
The main target users are workers and those responsible for occupational safety and health. The ICSC project is a common undertaking between the World Health Organization WHO and. If 156 g of sodium nitrate react and 112 g of sodium nitrite are recovered what is the percentage yield.
Question bf4d3 When 830x10-1 g of silver nitrate is dissolved in water and then mixed with excess potassium iodide solution 60000x10-1 g of precipitate form. Polyacrylamide-2-acrylamide-2-methylpropylsulfonate-dimethylidiallyl ammonium chloride sodium salt CAS Reg. 72275-68-4 Polyamides derived from reaction of one or more of the following acids with one or more of the following amines.
Azelaic acid Dimerized vegetable oil acids Amines. Bishexamethylene triamine and higher homologues. Nitrite NO 3 nitrate O 2 2.
Reaction ΔH kJ CH 4 g 2O g COg 2H O 8904. NaOHaq sodium hydroxide KOHaq potassium hydroxide CaOH 2 aq calcium hydroxide NH 3 aq aqueous ammonia Approximate Indicator pH Range Color for Color Change Change methyl orange 3144 red to yellow bromthymol blue 6076 yellow to blue phenolphthalein 89 colorless to. Une première étape où lon transforme lamine primaire en ion diazonium par laction de lacide nitreux.
Ce dernier étant particulièrement instable on utilise en fait le nitrite de sodium Na NO 2 en milieu acide. H 2 H 2 O. The BrattonMarshall reagent consists of two spray solutions.
The first is sodium nitrite in acid to effect the diazotization. And the second is a mainly ethanolic solution of N-1-naphthylethylenediamine dihydrochloride. This reagent is used specifically to visualize primary aromatic amines sulfonamides and urea and carbamate herbicides.
Paulys reagent is used to visualize phenols. At levels not to exceed 02 by weight of lubricants or release agents applied at levels not to exceed 1 lb. Per ton of finished paper or paperboard.
As an anticorrosion agent at levels not to exceed 02 by weight of wax emulsions used as internal sizing in the manufacture of paper and paperboard prior to. The lethal dose of sodium hypochlorite in humans has been reported to be about 200 mL of a solution containing 31 500-63 000 mgL although survival of patients who swallowed up to 1 L of a 525 corresponding to 52 500 mgL solution and about 500 mL of a 10 corresponding to 100 000 mgL sodium hypochlorite solution has been reported Racioppiet al 1994. Even in the case of misuse.
This website uses cookies to help provide you with the best possible online experience. Please read our Terms Conditions and Privacy Policy for information about. AJOGs Editors have active research programs and on occasion publish work in the Journal.
Editorauthors are masked to the peer review process and editorial decision-making of their own work and are not able to access this work in the online manuscript submission system. However the level of concentration strongly depends on the fermentation conditions of the cabbage. 30 According to Martinez-Villaluenga 31 producing cabbage at low-salt concentration improved ascorbigen content with the highest concentration being observed in low-sodium 05 NaCl sauerkraut produced from cabbage cultivated in winter using natural fermentation Table 4.
These reaction and degradation products are non-intentionally present in the plastic material NIAS. As far as they are relevant for the risk assessment the main reaction and degradation products of the intended application of a substance should be considered and included in the restrictions of the substance. However it is not possible to list and consider all reaction and degradation.