It is used extensively in paper manufacture as a binder for dyes and as a surface filler. The balanced chemical equation for this reaction is.

Aluminum and Hydrochloric acid react to form Aluminum Chloride and Hydrogen gas.
Aluminum sulfate acid reaction. An acidbase reaction is a type of chemical reaction that involves the exchange of one or more hydrogen ions H between species that may be neutral molecules such as water H 2 O or electrically charged ions such as ammonium NH 4. Or carbonate CO 3 2. It also includes similar processes that occur in molecules and ions that are acidic but do not donate.
Another major compound is aluminum sulfate a colourless salt obtained by the action of sulfuric acid on hydrated aluminum oxide. The commercial form is a hydrated crystalline solid with the chemical formula Al 2 SO 4 3. It is used extensively in paper manufacture as a binder for dyes and as a surface filler.
Aluminium aluminum in American and Canadian English is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals at approximately one third that of steel. It has a great affinity towards oxygen and forms a protective layer of oxide on the surface when exposed to air.
Aluminium visually resembles silver both in its color and. Aluminium chlorohydrate is a group of water-soluble specific aluminum salts having the general formula Al n Cl 3n-m mIt is used in cosmetics as an antiperspirant and as a coagulant in water purification. In water purification this compound is preferred in some cases because of its high charge which makes it more effective at destabilizing and removing suspended materials than other.
Baking powder contains baking soda sodium bicarbonate and a dry acid cream of tartar or sodium aluminum sulfate. When liquid is added to a baking recipe these two ingredients react to form bubbles of carbon dioxide gas. For iron-based deoxidizers ferric sulfate is present to oxidize the cuprous Cu 1 residue that develops on aluminum after alkaline etching to cupric Cu 2 sulfate.
The cuprous is insoluble whereas the cupric is soluble. As part of the reaction the soluble ferric will convert to the insoluble ferrous. The nitric present in the product is there to convert the ferrous back to ferric.
It has reactions as both a base and an acid. Aluminum oxide is insoluble in water and does not react like sodium oxide and magnesium oxide. The oxide ions are held too strongly in the solid lattice to react with the water.
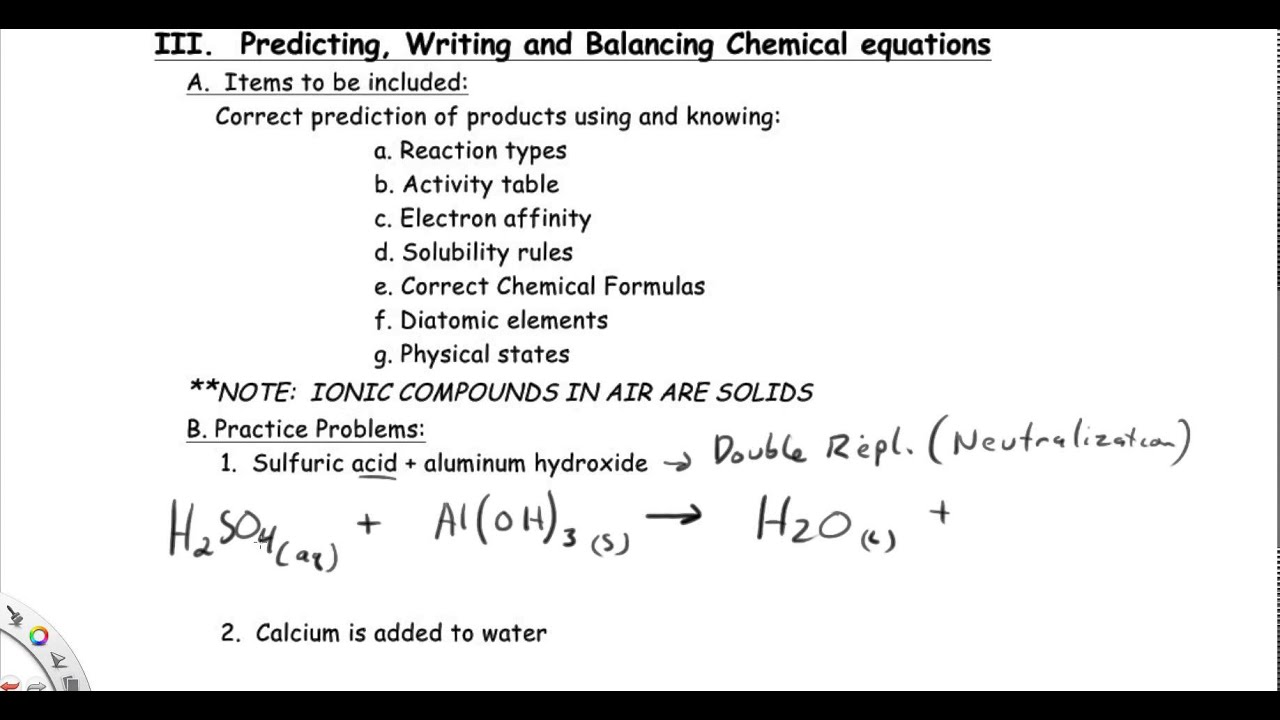
Aluminum oxide contains oxide ions and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium reacts with sulfuric acid h2so4 to produce aluminium sulfate al2so43 and hydrogen h2. Complete the balance the equation for this reaction.
The reaction with zinc indicates galvanized piping should not be used for sulfuric acid. Some metals are known to be actively corroded by sulfuric acid such as aluminum alloys and carbon steel and the rate of corrosion will actually increase with more dilute concentrations of H 2 SO 4 due to the increasing. The second step of the procedure is to convert the KAlOH 4 to alum by addition of sulfuric acid H 2 SO 4 in an acid-base reaction.
Under the experimental conditions the alum has a limited solubility in water and so it precipitates from the solution. The balanced chemical reaction that occurs in this step is. The overall balanced chemical reaction for the conversion of aluminum to alum.
Al 2 CO 3 3. Al 2 O 3. Aluminum and Hydrochloric acid react to form Aluminum Chloride and Hydrogen gas.
2 Al 6 HCl 3H 2 2 AlCl 3 Single Replacement 6. Calcium Hydroxide and Phosphoric acid react to form Calcium Phosphate and water. 3 CaOH 2 2 H 3 PO 4 Ca 3 PO 4 2 6H 2 O Double Replacement 7.
Copper and Sulfuric acid react to form Copper II Sulfate and water and Sulfur Dioxide Cu 2H 2 SO 4 CuSO 4 2. Aluminum hydroxide will decrease the level or effect of ferrous sulfate by increasing gastric pH. Applies only to oral form of both agents.
Ferrous sulfate will decrease the level or effect of bictegravir by cation binding in GI tract. Bictegravir and supplements containing iron can be taken together with food. The chemical equation for the reaction is.
KCl aq AgNO 3 aq AgCl s KNO 3 aq. M sulfuric acid solution. 12 Excess sodium hydroxide solution is added to 1500 mL of 0135 M aluminum chloride solution.
What mass of aluminum hydroxide is formed. T-31 13 What volume of 125 M sulfuric acid is needed to dissolve 0750 g of aluminum hydroxide. What is the molarity of the.
There is a reaction between sulfuric acid and sodium bicarbonate also called sodium hydrogen carbonate and baking soda. This reaction produces sodium sulfate carbon dioxide and water. The general equation for this reaction is.
Acid carbonate rarr salt carbon dioxide water. The balanced chemical equation for this reaction is. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way.
The primary aim of the cards is to promote the safe use of chemicals in the workplace. The main target users are workers and those responsible for occupational safety and health. The ICSC project is a common undertaking between the World Health Organization WHO and.
Sodium sulfatepotassium sulfatemagnesium sulfate. Sodium sulfatepotassium sulfatemagnesium sulfate increases toxicity of zoledronic acid by Other see comment. Coadministration with medications that cause fluid and electrolyte abnormalities may increase the risk of adverse events of seizure arrhythmias and.
00455 moles of hydrochloric acid. 12 moles of glucose C6H12O6 13. 032 moles of sodium bicarbonate.
Solve the following stoichiometry grams-grams problems. 1 The combustion of a sample of butane C4H10 lighter fluid produced 246 grams of water. 2 C4H10 13O2 —– 8CO2 10H2O a How many moles of water formed.
B How many moles of butane burned. The ferrous sulfate reaction is quicker since this salt disassociates into iron and sulfuric acid. The iron binds to the clay or precipitates out of the soil solution leaving the sulfuric acid.
Aluminum sulfate also acidifies soils but there are reports that it can be toxic to blueberries if high rates are applied. Many people want to change the soil pH right now. To do this you need to add.
How much sulfuric acid is needed to produce 28 moles of ironIII sulfate. Write the mole ratios for reactants in terms of products for the following equation. 2 Mg O 2 2 MgO 4.
How many moles of each reactant are needed to produce 25 mol of aluminum oxide by the following reaction. 4 Al 3 O 2 2 Al 2O 3 5. How many moles of each reactant would be necessary to produce 26 mol.
Aluminium nitrate was administered in the drinking water of four groups of 10 female SD rats for one month at the following doses. 0 375 750 or 1500 mgkg bwday. Food and water consumption and urine volume were measured daily.
Body weight and protein efficiency coefficients were calculated each week. On days 10 20 and 30 blood was analyzed. Copper sulfate is stable at normal temperatures.
When mixed with an acid copper sulfate will dissolve. However no products formed will be hazardous. Some people may exhibit a sensitivity to copper if copper sulfate makes contact with their skin.
Copper sulfate is a severe eye irritant and can cause substantial damage to the eyes. If inhaled the dust can cause respiratory. The result was achieved when the following conditions were applied.
A molar ratio between sodium sulfite and sodium dichromate equal to 6. Reaction time equal to 5 min before addition of sulfuric acid. PH of sodium dichromate solution equal to 2.
Summarizing there is an opportunity to utilize the dangerous wastes and reused them in the production scheme by minimizing or annulling the. Analytical 4 ACS reagent 2 BioReagent 2 Technique. Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within Products.
Hydrogen chloride solution Hydrochloric acid solution. Empirical Formula Hill Notation. Neutralization Reaction In these reactions acid and base reacts with each other and form salt and water.
For example hydrochloric acid reacts with sodium hydroxide base and forms sodium chloride salt and water. Single Displacement Reaction In these reactions more reactive metal displaces less reactive metal from its salt. In these reactions products can be determined through.