LiCIO3 lithium chlorate 10. Aluminium aluminum in American and Canadian English is a chemical element with the symbol Al and atomic number 13.

Sodium hydroxide in contact with acids and organic halogen compounds especially trichloroethylene may causes violent reactions.
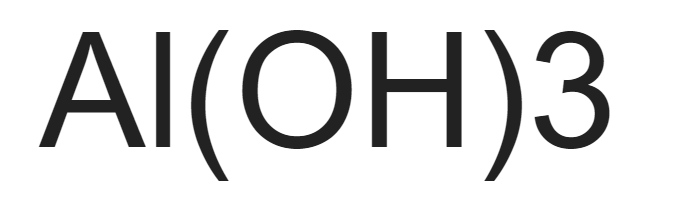
Aluminum hydroxide formula. Aluminum hydroxide is a basic inorganic salt that acts by neutralizing hydrochloric acid in gastric secretions. Aluminum hydroxide is slowly solubilized in the stomach and reacts with hydrochloric acid to form aluminum chloride and water. It also inhibits the action of.
The information below refers to products available in the United States that contain aluminum hydroxide. Products containing aluminum hydroxide. Amphojel Alternagel Alu-Cap Dialume Drug classes.
Antacids phosphate binders Aluminum hydroxide systemic is used in the treatment of. Aluminum hydroxide polymers and related species_____ 40 7. Rapidly reacting aluminum determined by ferron compared with calculated values for A13 and A10H2—__–___—– 43 8.
Computation of K_____ 49. CHEMISTRY OF ALUMINUM IN NATURAL WATER FORM AND STABILITY OF ALUMINUM HYDROXIDE COMPLEXES IN DILUTE SOLUTION By J. ROBERSON ABSTRACT Laboratory studies.
The alums double salts of formula MAlSO 4 2 12H 2 O where M is a singly charged cation such as K also contain the Al 3 ion. M can be the cation of sodium potassium rubidium cesium ammonium or thallium and the aluminum may be replaced by a variety of other M 3 ionseg gallium indium titanium vanadium chromium manganese iron or cobalt. The most important of such.
Antacids have 300600 mg aluminum hydroxide approximately 104. Aluminum is also found in soy-based infant formula 046093 mgL and milk-based infant formula 0058015 mgL. Birth defects We do not know if aluminum will cause birth defects in people.
Birth defects have not been seen in animals. Very young animals appeared weaker and less active in their cages and some. Aluminium aluminum in American and Canadian English is a chemical element with the symbol Al and atomic number 13.
Aluminium has a density lower than those of other common metals at approximately one third that of steel. It has a great affinity towards oxygen and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver both in its color and.
Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily.
Aluminium hydroxide is used widely in pharmaceutical and personal care products. Food related uses of aluminium compounds include preservatives fillers colouring agents anti-caking agents emulsifiers and baking powders. Soy-based infant formula can contain aluminium.
Natural aluminium minerals especially bentonite and zeolite are used in water purification sugar refining brewing and. Sodium hydroxide in contact with acids and organic halogen compounds especially trichloroethylene may causes violent reactions. Contact with nitromethane and other similar nitro compounds causes formation of shock-sensitive salts.
Contact with metals such as aluminum magnesium tin and zinc cause formation of flammable hydrogen gas. Aluminum hydroxideaspirincalcium carbonatemagnesium hydroxide systemic. Aspirin Buffered Arthritis Pain Formula Magnaprin Aspir-Mox Drug classes.
Salicylates Aluminum hydroxideaspirincalcium carbonatemagnesium hydroxide systemic is used in the treatment of. Al 2 O 3. Cr 2 H 8 N 2 O 7.
C 2 H 3 O 2 NH 4. NH 4 HCO 3. Ammonium carbonate formula NH 4 2 CO 3.
When the formula unit contains two or more of the same polyatomic ion that ion is written within parentheses and a subscript is written outside the parentheses to indicate the number of polyatomic ions. Parentheses and a subscript are not used unless more than one of a polyatomic ion is present in the formula unit eg calcium sulfate CaSO 4 not CaSO 4. Young and aged mice were subcutaneously injected with aluminum hydroxide AH CpG or AHCpG.
Twenty-four hours later draining lymph nodes dLNs were collected and RNA was extracted. A Weights of dLNs were measured and expressed as fold over contralateral PBS-injected dLNs. And respectively indicate P.
Which of these substances can interact with aluminum hydroxide Nac KOH HNO. Write the formula of the compound under the name 223. How many grams of lithium chloride must be taken to prepare 250 g solution with a mass fraction of 9 12 lithium chloride.
AlCl3 aluminum chloride 5. Ca3PO42 calcium phosphate 15. NaOH sodium hydroxide 6.
BaOH2 barium hydroxide 16. PbCl2 lead II chloride 7. Na2S sodium sulfide 17.
KNO3 potassium nitrate 8. Iron II chloride 18. MgOH2 magnesium hydroxide 9.
Na2CrO4 sodium chromate 19. LiCIO3 lithium chlorate 10. NH42SO4 ammonium sulfate 20.
NiS nickel II sulfide Write the chemical. Incompatibilities with Other Materials. Water metals acids aluminum zinc tin nitromethane leather flammable liquids organic halogens wool.
Toxic fumes of sodium oxide. Section 11 - Toxicological Information NIOSH RTECS CAS 1310-73-2 sodium hydroxide. The saturated solution of calcium hydroxide in water also reacts with and dissolves metals such as aluminum.
Calcium hydroxide also known as slaked lime with the chemical formula CaOH2 is a source of hydroxide ions when dissolved in aqueous solutions. Therefore this compound is a base. Due to electrolyte dissociation this compound liberates OH- ions.
How can calcium hydroxide be. Aluminum oxide reacts with sodium hydroxide to produce sodium aluminate and water. This reaction takes place at a temperature of 900-1100C.
A salt and water is obtained in this reaction in which. Ionic Compound Formula K sp. Aluminum hydroxide AlOH 3 1810 5.
Aluminum phosphate AlPO 4 6310 19. Barium carbonate BaCO 3 5110 9. Barium chromate BaCrO 4 1210 10.
Barium fluoride BaF 2 1010 6. Barium hydroxide BaOH 2 510 3. Barium sulfate BaSO 4 1110 10.
Barium sulfite BaSO 3 810 7. Barium thiosulfate BaS 2 O 3 1610 6. Sodium hydroxide 2 mgm3 Ceiling 10 mgm3 IDLH 2 mgm3 TWA Sodium carbonate none listed.
Molecular FormulaNaOH Molecular Weight 40 Section 10 - Stability and Reactivity Chemical Stability. Stable at room temperature in closed containers under normal storage and handling conditions. Moisture contact with water exposure to moist air or water prolonged exposure.
Lavilin is a leading international brand of aluminum-free deodorants and personal care products. Our company is rooted in the idea that ingredient quality is more important than profit which is why all of our products are free of aluminum and other harsh chemicals. NEW - Sport Deodorant.
1850 Womens. Helloyou are in right place to know about the reaction between sulfuric acid and sodium hydroxide. Sulfuric acid is an acid because it has hydrogen ionThe formula for sulfuric acid is H₂SO₄It is a strong acid.
Sodium hydroxide is a base because it has hydroxide ionThe chemical formula for sodium hydroxide is NaOHIt is a strong base. Chemical Formula Nomenclature Practice. Complete these in lab and on your own time for practice.
You should complete this by Sunday. Use the stock form for the transition metals. Give the formula for the following.
Sulfur dioxide SO2_ 2. Sodium thiosulfate ____Na2S2O3_____ 3. Synonyms Trade Names.
DOT ID Guide. 1823 154dry solid 1824 154solution Formula. 10 mgm 3 See.
NIOSH REL C 2 mgm 3 OSHA PEL TWA 2 mgm 3 See Appendix G. NMAM or OSHA Methods.