Acyl bromides and iodides. Aliphatic acyclic 32 and acyclic alcohols aldehydes ketones carboxylic acids and related esters lactones 33 ketals and acetals comprise more than 700 of the 1323 chemically defined flavoring 34 substances in the United States.
Chem publishes work from across the chemical sciences and at.
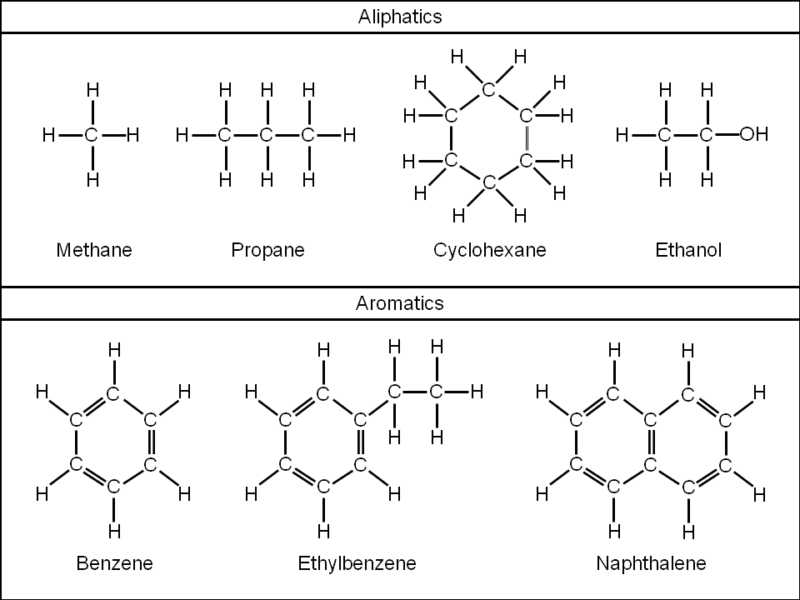
Aliphatic and aromatic. Hydroformylation of alkenes is the most important method of aliphatic formylation however it is largely restricted to an industrial setting due to the high temperatures and pressures involved. Several specialty methods exist for laboratory-scale synthesis including the Sommelet reaction Bouveault aldehyde synthesis or BodrouxChichibabin. An aliphatic compound is an organic compound containing carbon and hydrogen joined together in straight chains branched chains or non-aromatic rings.
It is one of two broad classes of hydrocarbons the other being aromatic compounds. Aromatic as well as aliphatic acyl fluorides are conveniently prepared directly from carboxylic acids using stable inexpensive commodity chemicals. PPh 3 NBS and Et 3 N-3HF in a bench-top protocol.
Cyanuric fluoride converts carboxylic acids to acyl fluorides. Acyl bromides and iodides. Acyl bromides and iodides are synthesized accordingly but are less common.
Acyl halides are. Aliphatic compound any chemical compound belonging to the organic class in which the atoms are connected by single double or triple bonds to form nonaromatic structures. One of the major structural groups of organic molecules the aliphatic compounds include the alkanes alkenes and alkynes and.
Due to the Thanksgiving Holiday observed in the US All orders placed on Wednesday 24th Thursday 25th Friday November 26th will ship out the week of November 29th. Simply put aliphatic compounds are compounds that do not incorporate any aromatic rings in their molecular structure. The following table lists the IUPAC names assigned to simple continuous-chain alkanes from C-1 to C-10.
A common ane suffix identifies these compounds as alkanes. Longer chain alkanes are well known and their names may be found in many reference and text books. These hydrocarbons contain carbon and hydrogen attached in a ring system with delocalized pi electrons.
These hydrocarbons contain carbon and hydrogen attached in straight chains branched chains or in non aromatic ring forms. They do not show aromaticity. All aromatic compounds follow Huckels rule.
The meaning of aromatic is of relating to or having aroma. How to use aromatic in a sentence. Synonym Discussion of Aromatic.
Tryptophan W one of 3 Ts with a Y so it is aromatic will tryp you up because it is hard to remember has a 3 carbon start to N or indole ring on methylene Aliphatic hydroxyl C H NH2 HO CH2 COOH Serine S hydroxyl alanine CH3 C C COOH OH H H NH2 Threonine T one of 3. The carbon compounds are related in a straight chain way in aliphatic compounds. In aromatic compounds the carbon compounds are associated with conjugated pi electrons in the manner of a ring structure.
Are all aromatic compounds cyclic. Cyclic compounds may or may not be aromatic. Benzene is an example of a cyclic aromatic compound while cyclohexane is non-aromatic.
Environment Coupling Value Hz Aliphatic C-H. Aliphatic CX-H XNOS 1 J CH. Environment Coupling Value Hz C-F.
611 Aromatic Compounds There are two major classes of organic chemicals aliphatic. Straight or branched chain organic substances aromatic or arene. Includes one or more ring of six carbon atoms with delocalised bonding.
All of the organic substances we have looked at so far have been aliphatic Benzene belongs to the aromatic class. Benzenes Structure The simplest arene is benzene. Older chemists classified hydrocarbons as either aliphatic or aromatic.
The classification was done based on their source and properties. As such it was found out that Aliphatic hydrocarbons were derived from chemical degradation of fats or oils whereas aromatic hydrocarbons contained substances that were a result of chemical degradation of certain plant extracts. However today we classify.
The terms aliphatic and aromatic are retained in modern terminology but the compounds they describe are distinguished on the basis of structure rather than origin. Aliphatic hydrocarbons are divided into three main groups according to the types of bonds they contain. Alkanes alkenes and alkynes.
Alkanes have only single bonds alkenes contain a carbon-carbon double bond and alkynes contain. It assumes that you are reasonably confident about naming compounds containing chains of carbon atoms aliphatic compounds. If you arent sure about naming aliphatic compounds follow this link before you go on.
Naming aromatic compounds isnt quite so straightforward as naming chain compounds. Often more than one name is acceptable and its not uncommon to find the old names. What is ethoxylation of alcohol.
Alcohol ethoxylates belong to the class of compounds which are synthesized via the reaction of a fatty alcohol and ethylene oxide resulting in a molecule that consists of two parts one a carbon-rich fatty alcohol and the second part a hydrophilic polyoxyethylene chain. This dual structural aspect of ethoxylated alcohol containing a hydrophobic portion. The non-bonding electron pair on the nitrogen is not part of the aromatic π-electron sextet and may bond to a proton or other electrophile without disrupting the aromatic system.
In the case of thiophene a sulfur analog of furan one of the sulfur electron pairs colored blue participates in the aromatic ring π-electron conjugation. The last compound is imidazole a heterocycle having two. Aliphatic acyclic 32 and acyclic alcohols aldehydes ketones carboxylic acids and related esters lactones 33 ketals and acetals comprise more than 700 of the 1323 chemically defined flavoring 34 substances in the United States.
Additional structural categories include aromatic. Total aliphatic and indole glucosinolates were significantly modified by all cooking treatments but not by steaming. In general the steaming led to the lowest loss of total glucosinolates while stir-frying and stir-fryingboiling presented the highest loss.
Stir-frying and stir-fryingboiling the two most popular methods for most homemade dishes in China cause great losses of chlorophyll. Unlike aliphatic organics nomenclature of benzene-derived compounds can be confusing because a single aromatic compound can have multiple possible names such as common and systematic names be associated with its structure. In these sections we will analyze some of.
22 Raman Bands υCC alicyclic aliphatic chain vibrations 600 - 1300 cm-1 medium Medium υCS 1000 - 1250 cm-1 strong weak υCC aromatic ring chain vibrations 1580 1600 cm-1 strong medium 1450 1500 cm-1 medium medium 1000 cm-1 strongmedium weak δCH3 1380 cm. Povinal for aliphatic aromatic and chlorinated solvents. Highly flexible excellent for hose pumps.
Vytex for food beverage and general purpose applications. Smooth internal bore easy cleaning. T-Alimen for food and beverage grade tubing.
Chem a sister journal to Cell provides a home for seminal and insightful research and showcases how fundamental studies in chemistry and its sub-disciplines may help in finding potential solutions to the global challenges of tomorrow. Chem publishes work from across the chemical sciences and at. GacoFlex UB64 GacoFlex UB64C GacoFlex U64 GacoFlex U64C GacoFlex U66 GacoFlex U66C GacoFlex U61 GacoFlex U91 Aliphatic Urethanes.
GacoFlex E5320 GacoFlex E5691. The test is positive for aliphatic aldehydes but is often indecisive for aromatic aldehydes where Jones Reagent is often useful see 5. B Tollens reagent Ammonical silver nitrate solution Aldehydes are readily oxidised to carboxylic acids and will reduce Tollens reagent to produce a silver mirror on the inside of a clean test tube.