We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. In this case excess dichromate will further oxidize the aldehyde to a carboxylic acid so either the aldehyde is distilled out as it forms if volatile or milder reagents such as PCC are used.

Identify the organic class to which the compound belongs.
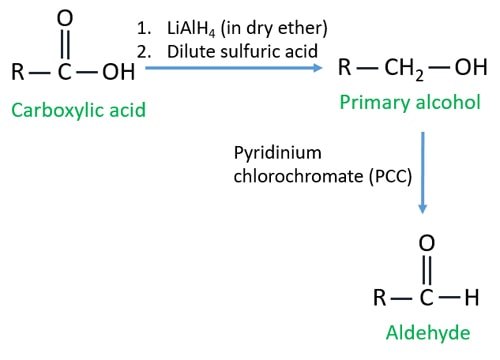
Aldehyde to acid. In this case excess dichromate will further oxidize the aldehyde to a carboxylic acid so either the aldehyde is distilled out as it forms if volatile or milder reagents such as PCC are used. O CH 3 CH 2 9 OH CH 3 CH 2 8 CHO H 2 O. Oxidation of primary alcohols to form aldehydes can be achieved under milder chromium-free conditions by employing methods or reagents such as IBX.
Identify the organic class to which the compound belongs. Show all questions. Standards Cyclohexanone Benzophenone and Benzaldehyde.
Procedure Add a solution of 1 or 2 drops or 30 mg of unknown in 2 mL of 95 ethanol to 3 mL of 24-dinitrophenylhydrazine reagent. Shake vigorously and if no precipitate forms immediately allow the solution to stand for 15 minutes. The 24-dinitrophenylhydrazine reagent will already be prepared for.
Cyclamen Aldehyde is a very powerful floral green note used in many perfumery applications to blend into fresh floral green or ozonicmarine accords. It is an ingredient with growing use and importance in helping to build replacement accords for materials disappearing from the Perfumers palette. Cyclamen has good overall stability except in extremes of pH.
It has good tenacity and. Upon first inspection a typical infrared spectrum can be visually divided into two regions. The left half above 2000 cm-1 usually contains relatively few peaks but some very diagnostic information can be found hereFirst alkane C-H stretching absorptions just below 3000 cm-1 demonstrate the presence of saturated carbons and signals just above 3000 cm-1 demonstrate unsaturation.
Aldehyde and Ketone reduction by LiAlH 4 to Alcohols. LiAlH 4 is a strong reduction reagent used in organic chemistry. LiAlH 4 can reduce aldehyde and ketone to alcohols.
When aldehyde is reduced by LiAlH 4 primary alcohol is given as the productBut reduction of ketone will give a secondary alcohol. Product lines include aldehyde group amine group potassium bromide inhibitors catalysts and ligands PEG linkers and other building blocks. Products Aldehydes Imidazoles Pyridines Amines Indole and Oxindoles Sulfonamines Boronic Acids Iodos Thiazoles Bromides Nitro Compounds Other Heterocycles Carboxes.
The primary enzymes involved in alcohol metabolism are alcohol dehydrogenase ADH and aldehyde dehydrogenase ALDH. Both enzymes occur in several forms that are encoded by different genes. Moreover there are variants ie alleles of some of these genes that encode enzymes with different characteristics and which have different ethnic distributions.
Glyoxylic acid is a component of the HopkinsCole reaction used to check for the presence of tryptophan in proteins. Glyoxylic acid is one of several ketone- and aldehyde-containing carboxylic acids that together are abundant in secondary organic aerosols. Ammonia is known to behave as a weak base since it combines with many acids to form salts.
For example when it is reacted with hydrochloric acid ammonia is converted into ammonium chlorideAll the salts that are produced from such acid-base reactions are known to contain the ammonium cation denoted by NH 4 It is interesting to note that ammonia also exhibits weak acidic qualities and can. Since an aldehyde carbonyl group must always lie at the end of a carbon chain it is by default position 1 and therefore defines the numbering direction. A ketone carbonyl function may be located anywhere within a chain or ring and its position is given by a locator number.
Chain numbering normally starts from the end nearest the carbonyl group. In cyclic ketones the carbonyl group is. In aldehydes at least one bond on the carbonyl group is a carbon-to-hydrogen bond.
In ketones both available bonds on the carbonyl carbon atom are carbon-to-carbon bonds. Aldehydes are synthesized by the oxidation of primary alcohols. The aldehyde can be further oxidized to a carboxylic acid.
Ketones are prepared by the oxidation of secondary. The addition of water to an aldehyde results in the formation of a hydrate. The formation of a hydrate proceeds via a nucleophilic addition mechanism.
Water acting as a nucleophile is attracted to the partially positive carbon of the carbonyl group generating an oxonium ion. The oxonium ion liberates a hydrogen ion that is picked up by the oxygen anion in an acidbase reaction. Aldehyde und Ketone sind wichtige Verbindungsklassen der organischen Chemie.
Die wichtigsten Vertreter sind die Alkanale und Alkanone. Als funktionelle Gruppe enthalten die Moleküle beider Stoffklassen die Carbonylgruppe -COAldehyde und Ketone zeichen sich u. Durch intensive Grüche aus.
Sie finden deshalb Anwendung als Geruchs- und Aromastoffe. The 40 solution of formaldehyde forms Formalin which is used in preserving biological specimens. It is also used in embalming tanning preparing glues and polymeric products as germicides insecticides fungicides for plants drug testing and photography etc.
Acetaldehyde used in the production of acetic acid and pyridine derivativesBenzaldehyde is used in perfumes cosmetic products and. This aldehyde is highly reactive. MeCN 01 acetic acid.
Kidney dissociation for scRNA-seq. Kidneys were placed into a small tube containing 1. The aldehyde is oxidized to an acid as an intermediate through the conversion of NAD to NADH H.
Then an inorganic phosphate is added in a phosphate esteer synthesis. This and all remaining reactions occur twice for each glucose-6-phosphate six carbons since there are now two molecules of 3-carbons each. This reaction is catalyzed by glyceraldehyde-3-phosphate.
Cleaner liquid citric acid renewable renewable Ingredients containing more than 50 carbon from renewable sources. Aldehyde C18 So-Called Product No. 104-61-0 Product Aldron Product No.
Reaction of ethanoic acid and ethanol in closed system. After sometime ethanoic acid and ethanol are mixed in a closed system this mixture come to the equilibrium. In that mixture ethanoic acid ethanol ethyl ethanoate and water exist as chemical compounds.
This system is a liquid phase equilibrium. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production.
A second enzyme aldehyde dehydrogenase ALDH and NAD convert acetaldehyde to acetic acid generating even more NADH. In these reactions the coenzyme NAD is reduced to NADH and alcohol and acetaldehyde are oxidized. Review the oxidation of alcohol by alcohol dehydrogenase ADH.
Official website for Google search engine. Search for web content images videos news and maps. Log in for access to Gmail and Google Drive.
Find Android apps using Google Play.