Dose Modifications Monotherapy Febrile neutropenia ANC 500mm 3 for 1 week other grade 34 nonhematological toxicities severecumulative cutaneous reactions withhold treatment. Consider a starting dose of thalidomide 100 mg daily and increase as tolerated.

Moderate Citalopram causes dose-dependent QT interval prolongation.
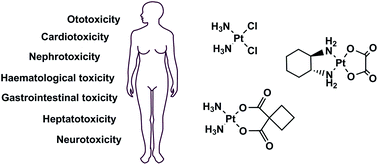
Adverse effect of cisplatin. Cisplatin interacts with DNA and forms covalent adduct with purine DNA bases and this platinum compound interaction is the root cause for cytotoxic effect of cisplatin Yousef Saad et al 2009. Cisplatin treatment has been associated with several toxic side effects including nephrotoxicity de Jongh van Veen et al 2003 hepatotoxicity and Cardiotoxicity Al-Majed 2007. The carcinogenic effect of cisplatin was studied in BD IX rats.
Cisplatin was administered intraperitoneally ip to 50 BD IX rats for 3 weeks 3 X 1 mgkg body weight per week. Four hundred and fifty -five days after the first application 33 animals died 13 of them related to malignancies. 12 leukemias and 1 renal fibrosarcoma.
The development of acute leukemia coincident with the use of. In our previous chemotherapy trial which investigated docetaxel plus cisplatin plus fluorouracil 7 a high incidence of grade 3 or 4 acute adverse events. This effect is apparently cell-cycle nonspecific.
The aquation of carboplatin which is thought to produce the active species occurs at a slower rate than in the case of cisplatin. Despite this difference it appears that both carboplatin and cisplatin induce equal numbers of drug-DNA cross-links causing equivalent lesions and biological effects. The differences in potencies for carboplatin.
The platinum-based drugs cisplatin carboplatin and oxaliplatin are regularly prescribed in the treatment of cancer and while they are effective their use is limited by their severe dose-limiting side effects also referred to as adverse effectsevents. In total a cancer patient can experience any combination of around 40 specific side effects. The dose-limiting side effect for cisplatin.
Also indicated in combination with cisplatin for the treatment of unresectable locally advanced or metastatic NSCLC in patients who have not previously received chemotherapy. 75 mgm 2 IV over 1 hr q3Weeks. Dose Modifications Monotherapy Febrile neutropenia ANC 500mm 3 for 1 week other grade 34 nonhematological toxicities severecumulative cutaneous reactions withhold treatment.
Amikacin and cisplatin both increase nephrotoxicity andor ototoxicity. Clarithromycin will increase the level or effect of amikacin by P-glycoprotein MDR1 efflux transporter. The total dose of cisplatin may be fractionated and given over 3 days.
Substituting oral etoposide for intravenous etoposide is NOT recommended in this regimen. Substituting cisplatin with carboplatin in patients judged unsuitable for cisplatin may be considered however cisplatin is the preferred agent with this protocol. Consider a starting dose of thalidomide 100 mg daily and increase as tolerated.
Thalidomide may be dose reduced or omitted. The schedule presented differs from the administration as per original study by Lee et al r in which the daily dose of cisplatin cyclophosphamide and etoposide was combined in a 1-L bag of 09 normal saline and doxorubicin was infused separately in more than. Moderate Closely monitor renal function and audiometric testing if concomitant use of cisplatin and furosemide is necessary.
Both cisplatin and furosemide can cause nephrotoxicity and ototoxicity which may be additive when used together. Moderate Citalopram causes dose-dependent QT interval prolongation. No adverse developmental effects from mannitol were reported in animal studies.
However fluid shifts occurred in fetal sheep in response to maternal infusion of mannitol33007 There are no available human data regarding inhaled mannitol to evaluate a drug-associated risk for major birth defects miscarriage or other adverse maternal or fetal outcomes50722 Based on animal reproduction. The resultant effect in this instance is increased corticosteroid exposure which may manifest as adrenal suppression andor weight gain. It is important for clinicians to identify and understand the clinical relevance of drug interactions with OIMs to better assist patients and improve therapeutic outcomes.
Accordingly this review was undertaken to provide health care providers with a. The European Journal of Cancer EJC integrates preclinical translational and clinical research in cancer from epidemiology carcinogenesis and biology through to innovations in cancer treatment and patient careThe journal publishes original research reviews previews editorial comments and correspondence. The EJC is the official journal of the European Organisation for Research and.
In a murine model taurine exerted cardioprotective effects against cisplatin-induced cardiotoxicityChowdhury 2016. Limited clinical studies have demonstrated a positive effect of taurine in hypertension. Studies used supplemental taurine 3 to 6 gday treatment duration range 1 to 8 weeksAbebe 2011 Wójcik 2010 Yamori 2010 Limited studies with small numbers of.
Vatalanib INN codenamed PTK787 or PTKZK is a small molecule protein kinase inhibitor that inhibits angiogenesisIt is being studied as a possible treatment for several types of cancer particularly cancer that is at an advanced stage or has not responded to chemotherapyVatalanib is orally active that is it is effective when taken by mouth. Anthracyclines is a class of drugs used in cancer chemotherapy that are extracted from Streptomyces bacterium. These compounds are used to treat many cancers including leukemias lymphomas breast stomach uterine ovarian bladder cancer and lung cancersThe first anthracycline discovered was daunorubicin trade name Daunomycin which is produced naturally by Streptomyces peucetius a.