Molecular formula of acetylsalicylic acid aspirin C 9 H 8 O 4. The German chemist Felix Hoffman.

Diacetylmorphine also known as.
Acetylsalicylic acid to salicylic acid mechanism. Salicylic acid is an organic compound with the formula HOC 6 H 4 CO 2 H. A colorless bitter-tasting solid it is a precursor to and a metabolite of aspirin acetylsalicylic acid. It is a plant hormone.
The name is from Latin salix for willow treeIt is an ingredient in some anti-acne productsSalts and esters of salicylic acid are known as salicylates. Aspirin also known as acetylsalicylic acid ASA is a medication used to reduce pain fever or inflammation. Specific inflammatory conditions which aspirin is used to treat include Kawasaki disease pericarditis and rheumatic fever.
Aspirin given shortly after a heart attack decreases the risk of death. Aspirin is also used long-term to help prevent further heart attacks ischaemic. Acetylsalicylic acid ASA is a potent irreversible inhibitor of platelet aggregation but loses its action after first-pass deacetylation to salicylic acid SA.
Acetylsalicylic acid was launched into the pharmacy industry more than 100 years ago. While initially conceived as an analgesic doctors soon discovered that it had many other medicinal benefits. The German chemist Felix Hoffman.
Acetylsalicylic acid ASA blocks prostaglandin synthesis. It is non-selective for COX-1 and COX-2 enzymes 91011. Inhibition of COX-1 results in the inhibition of platelet aggregation for about 7-10 days average platelet lifespan.
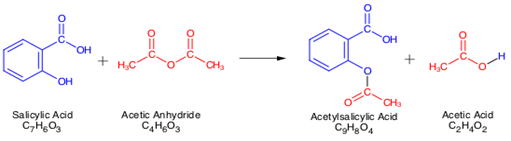
The acetyl group of acetylsalicylic acid binds with a serine residue of the cyclooxygenase-1 COX-1 enzyme leading to irreversible. In this reaction salicylic acid is reacted with acetic anhydride in an acidic medium which leads to the acetylation of the hydroxyl group present in the salicylic acid thereby resulting in the production of acetylsalicylic acid aspirin. Acetic acid is produced as a by-product of this reaction which is also present as one of the impurities during large scale production of aspirin and has.
Acetylsalicylic acid ASA which is being sold under the trade name aspirin by the Bayer Company is synthesized by the conversion of Salicylic acid SA to Acetylsalicylic acid ASA using acetic anhydride in the presence of phosphoric acid acting as a catalyst. The reaction involves the conversion of a phenol to an ester. This reaction is.
Acetylsalicylic acid was administered orally in a single dose or in repeated doses at different times before legal interruption. The mean passage rates were about 6-15. They were independent of the maternal serum concentrations of salicylic acid.
The distribution of salicylic acid on the fetal liver intestine kidneys lungs and brain was. Mechanism of action. Salicylic acid directly irreversibly inhibits COX-1 and COX-2 to decrease conversion of arachidonic acid to precursors of prostaglandins and thromboxanes.
Salicylates use in rheumatic diseases is due to its analgesic and anti-inflammatory activity. Salicylic acid is a key ingredient in many skin-care products for the treatment of acne psoriasis calluses corns. Salicylic acid the main metabolite of aspirin is an integral part of human and animal metabolism.
While in humans much of it is attributable to diet a substantial part is synthesized endogenouslyAspirin is part of a group of medications called nonsteroidal anti-inflammatory drugs NSAIDs but differs from most other NSAIDs in the mechanism of action. Though it and with similar structure. Salicylic acid is an intriguing ingredient being a chemical precursor to acetylsalicylic acid more commonly known as aspirin.
The watery bark sap of the willow tree Salix alba contains high concentrations of salicylic acid which can be refined to make aspirin. Here limiting reagent is salicylic acid. Hence yield should be calculated from its amount taken.
Molecular formula of salicylic acid C 7 H 6 O 3. Molecular formula of acetylsalicylic acid aspirin C 9 H 8 O 4. Molecular weight of salicylic acid 138 gmole.
Molecular weight of acetyl salicylic acid aspirin 180 gmole. Salicylic acid as a peeling agent has a number of indications including acne vulgaris melasma photodamage freckles and lentigines. The efficacy and safety of salicylic acid peeling in Fitzpatrick skin types IIII as well as in skin types V and VI have been well documented in the literature.
This paper reviews the available data and literature on salicylic acid as a peeling agent and its. To synthesize salicylic acid methyl salicylate limiting reagent is added to sodium hydroxide and heated. In this reaction the hydride ion acts as the nucleophile attacking the carbonyl carbon and replacing the methoxy group.
Once the reaction is complete acid is added to the mixture to protonate the carboxylate ions to produce the acid. 1 Acetylsalicylic acid aspirin retains the carboxyl group COOH of salicylic acid and makes a substitution in the hydroxyl group OH. The drug was developed at Bayer by Felix Hoffmann.
Acetylation made aspirin more tolerable to the gastrointestinal tract which led to widespread use. Salicylic acid is a benzene ring with a phenol HO group and a carboxylic acid COOH group. Whereas other scientists had focused on the carboxylic acid group Dr Felix Hoffman a German chemist at Friedrich Bayer and Co concentrated on the phenol group and managed on August 10 th 1897 to acetylate the phenol group and produce pure stable acetylsalicylic acid ASA for the first time 12.
Aspirin hay acetylsalicylic acid ASA acetosal là một dẫn xuất của acid salicylic thuộc nhóm thuốc chống viêm non-steroid. Có tác dụng giảm đau hạ sốt chống viêm. Nó còn có tác dụng chống kết tập tiểu cầu khi dùng liều thấp kéo dài có thể phòng ngừa đau tim và hình thành cục nghẽn trong mạch máu.
Salicylic acid could be displaced from protein binding sites or it could itself displace other protein-bound drugs and result in an enhanced effect of the displaced drug. Piroxicam aspirin will increase the level or effect of piroxicam by acidic anionic drug competition for renal tubular clearance. The acetylsalicylic acid soon to be known as aspirin was put through clinical trials by Dresers pharmacology division.
Initial reports were that it was a successful antipyretic but Dreser rejected it on the grounds that it may cause tachycardia and palpations. Ten days after his discovery of acetylsalicylic acid Hoffmann produced a second famous drug. Diacetylmorphine also known as.