The increased pH and larger surface area of the small intestine causes aspirin to be absorbed more slowly there as more of it is ionized. A second dosage may be necessary 6 hours after the first.
By GAVIN THOMAS October 28 2019 1230 am.
Acetylsalicylic acid a weak acid. Aspirin acetylsalicylic acid is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Aspirin is a weak acid that is only slightly soluble in water. Aspirin can be prepared by reacting salicylic acid and acetic anhydride in the presence of an acid catalyst.
Structure of Aspirin acetylsalicylic. Acetylsalicylic acid is a weak acid and very little of it is ionized in the stomach after oral administration. Acetylsalicylic acid is quickly absorbed through the cell membrane in the acidic conditions of the stomach.
The increased pH and larger surface area of the small intestine causes aspirin to be absorbed more slowly there as more of it is ionized. Owing to the formation of concretions. In contrast a weak acid only partially dissociates and at equilibrium both the acid and the conjugate base are in solution.
Examples of strong acids are hydrochloric acid HCl hydroiodic acid HI hydrobromic acid HBr perchloric acid HClO 4 nitric acid HNO 3 and sulfuric acid H 2 SO 4. In water each of these essentially ionizes 100. The stronger an acid is the more easily it.
A weak acid is any acid that reacts with water donates H ions to a very small extent usually less than 5 - 10. An aqueous solution of a weak acid in a state of equilibrium would consist mainly of the unionized form of the acid and only a small amount of hydronium ions and of the anion conjugate base of the weak acid. The equation representing the ionization of any weak acid HA and.
Answer 1 of 3. Acetil salicylic acid is an organic acid having one carboxilic group which confers the acidity to the acetil salicylic acid and then we can write it as C8H7COOH then a neutralization reaction of the acid with soda NaOH will be C8H7COOH NaOH C8H7COONa H2O or C9H8O4 Na. Answer 1 of 3.
The neutralising reaction can produce two salts - either the monosodium variety generally known simply as sodium salicylate where the proton from the carboxylic acid group is donated or the disodium one where both available protons are donated. Acidbase reactions are essential in both biochemistry and industrial chemistry. Moreover many of the substances we encounter in our homes the supermarket and the pharmacy are acids or bases.
For example aspirin is an acid acetylsalicylic acid and antacids are bases. In fact every amateur chef who has prepared mayonnaise or squeezed a. 1-3 is a Strong Acid 4-7 is a Weak Acid 7 is Neutral 8-10 is a Weak Alkali 11-14 is a Strong Alkali.
Examples of acids in everyday life. Examples of bases and alkalis in everyday life. Vinegar is diluted acetic acid which is what gives salad dressings and pickled vegetables their tart taste.
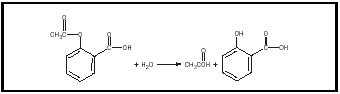
Oranges lemons and limes contain citric acid which gives them their sour taste. Acetylsalicylic Acid is composed from salicylic acid reacted with acetic anhydrates its reaction turns salicylic acid that first in hydroxyl group change to ester group R-OH into R-OCOCH 3. Acetylsalicylic Acid is white crystal and categorized as a weak acid.
Acetylsalicylic Acid often smell like those vinegar. It is easy to decompose through hydrolysis. Its decomposed rapidly when reacted.
Synthesis of thin film nanocomposite MCM-41NH2 with chitosan using a green simple method and expending the least energy is described. Ankylosis of primary teeth is most frequently observed in In neurons glutamate is an amino acid that binds only to The descending or repolarizing phase of the action potential is caused by GABA is aan The risk of a health care worker contracting hepatitis B through a needlestick injury from a chronic hepatitis B carrier is increased when the patients serology report indicates the. Thus acetylsalicylic acid aspirin has weak native fluorescence but its base-hydrolysis conjugate the salicylate ion strongly fluoresces at 400 nm after it has been excited at about 310 nm.
This property has been used to determine aspirin and salicylates directly in serum urine and plasma samples. In the same way hydrolysis has been successfully applied to the determination of. The risk or severity of adverse effects can be increased when Acetylsalicylic acid is combined with Alendronic acid.
The risk or severity of myopathy rhabdomyolysis and myoglobinuria can be increased when Alendronic acid is combined with Acipimox. The risk or severity of nephrotoxicity and hypocalcemia can be increased when Acyclovir is combined. The risk or severity of adverse effects can be increased when Acetylsalicylic acid is combined with Ursodeoxycholic acid.
The risk or severity of bleeding and bruising can be increased when Alteplase is combined with Ursodeoxycholic acid. Aluminium phosphate can cause a decrease in the absorption of Ursodeoxycholic acid resulting in a. C 4 H 4 N 2 O.
Examples of weak Acids. By GAVIN THOMAS October 28 2019 1230 am. Examples of strong acids.
By GAVIN THOMAS October 28 2019 1216 am. By GAVIN THOMAS October 28 2019 1209 am. Examples of Monocarboxylic Acid.
By GAVIN THOMAS October 28 2019 1204 am. Examples of Organic Acids. By GAVIN THOMAS.
AUS-e-TUTE is a science education website providing notes quizzes tests exams games drills worksheets and syllabus study guides for high school science students and teachers. Contains 325 mg of aspirin acetylsalicylic acid 1000 mg of citric acid and 1916 mg of sodium bicarbonate. The acids originally contained in a tablet give only 174 mmol of H which is not enough to neutralize all of the sodium bicar-bonate 228 mmol.
If students start by dissolving the tablet in pure water the sodium bicarbonate is in excess and the acid H is the limiting reactant. Acetylsalicylic acid aspirin HC 9 H 7 O 4 is a weak acid with Ka 275x10 5 at 25 C. 300 g of sodium acetylsalicylate NaC 9 H 7 O 4 is added to 2000 mL of 0100 M solution of this acid.
Calculate the pH of the resulting solution at 25 C. Molarity of NaC H O 300 g 9 7 4 1 mol x 202 g 1 x 00743 M 0200 L HC 9 H 7 O 4 H 2. Aspirin is a weak acid that also undergoes slow hydrolysis.
Ie each aspirin molecule reacts with two hydroxide ions. To overcome this problem a known excess amount of base is added to the sample solution and an HCl titration is carried out to determine the amount of unreacted base. This is subtracted from the initial amount of base to find the amount of base that actually reacted with the.
Metabolic acidosis develops when the amount of acid in the body is increased through ingestion of a substance that is or can be broken down metabolized to an acidsuch as wood alcohol methanol antifreeze ethylene glycol or large doses of aspirin acetylsalicylic acid. Many other drugs and poisons can cause acidosis. Aspirin chemically known as acetylsalicylic acid is a well-known analgesic and antipyretic substance causing irreversible inhibition of prostaglandin synthesis.
Being a salicylate SA also has anti-inflammatory properties. The concentration at which the anti-inflammatory action of SA is most pronounced is between 05 and 5 ww. In addition the anion gap should always be adjusted for the albumin concentration because this weak acid may account for up to 75 of the anion gap.
363940 Without correction for. With a half-life of just 30 minutes it would be necessary for you to take a single dosage anywhere between 324mg to 1000mg to relieve pain. A second dosage may be necessary 6 hours after the first.
The maximum dosage of aspirin in a single day is 4000mg. Tryptophan is the least plentiful of all 22 amino acids and an essential amino acid in humans provided by food Tryptophan is found in most proteins and a precursor of serotoninTryptophan is converted to 5-hydroxy-tryptophan converted in turn to serotonin a neurotransmitter essential in regulating appetite sleep mood and painTryptophan is a natural sedative and present in dairy. The active ingredient in aspirin acetylsalicylic acid causes many biochemical changes in the body including acting as a blood thinner.
The blood thinner part is what caught my attention as that is also the primary function of many commercial rat poisons including Warfarin. The MSDS for acetylsalicylic acid gives an LD50 of 1500 mgkg which makes this look like a promising poison.