Aspirin is an orally administered non-steroidal antiinflammatory agent. 57 - 218 - 221.
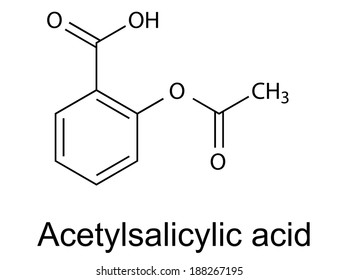
Acetylsalicylic acid Commonly known or available as Aspirin DrugBank Accession Number DB00945 Background.
Acetyl salicylic acid chemical formula. Acetylsalicylic acid commonly known as Aspirin is a prototypical analgesic with a chemical formula C 9 H 8 O 4. It is also known as aspirin or 2-Acetoxybenzoic acid. It appears as a crystalline powder which is colourless to white.
Generally it has no smell but when in moist air it acquires a smell of acetic acid. It has a flashpoint of 482 F. It is most widely used in medication to treat.
Acetylsalicylic acid was first produced by Alsatian Chemist Charles Frederic Gerhardt in 1853 from sodium salicylate and acetyl chloride. Soon the drug became famous and Bayer a drug and dye firm started producing it at large scale. Bayer named it Aspirin.
It was Bayers brand name for the drug. Its popularity declined in 1962 after the synthesis of drugs like paracetamol and ibuprofen. Aspirin is an orally administered non-steroidal antiinflammatory agent.
Acetylsalicylic acid binds to and acetylates serine residues in cyclooxygenases resulting in decreased synthesis of prostaglandin platelet aggregation and inflammation. This agent exhibits analgesic antipyretic and anticoagulant properties. In some countries this medicine may only be approved for veterinary use.
In the US Acetylsalicylic Acid is a member of the following drug classes. Platelet aggregation inhibitors salicylates and is used to treat Angina Angina Pectoris Prophylaxis Ankylosing Spondylitis Antiphospholipid Syndrome Aseptic Necrosis Back Pain Fever Heart Attack Ischemic Stroke. Aspirin also known as acetylsalicylic acid ASA is a medication used to reduce pain fever or inflammation.
Specific inflammatory conditions which aspirin is used to treat include Kawasaki disease pericarditis and rheumatic fever. Aspirin given shortly after a heart attack decreases the risk of death. Aspirin is also used long-term to help prevent further heart attacks ischaemic.
Aspirin is a salicylate drug often used as an analgesic to relieve minor aches and pains as an anti-inflammatory compound that inhibits Cox-1Target. Cox-1Aspirin USAN also known as acetylsalicylic acid is a salicylate drug often used as ananalgesic to relieve minor aches and pains as an antipyretic to reduce fever and as an anti-inflammatory medication. The active ingredient of.
Acetylsalicylic acid Commonly known or available as Aspirin DrugBank Accession Number DB00945 Background. Also known as Aspirin acetylsalicylic acid ASA is a commonly used drug for the treatment of pain and fever due to various causes. Acetylsalicylic acid has both anti-inflammatory and antipyretic effects.
This drug also inhibits platelet aggregation and is used in the prevention of blood. Salicylic acid is an organic compound with the formula HOC 6 H 4 CO 2 H. A colorless bitter-tasting solid it is a precursor to and a metabolite of aspirin acetylsalicylic acid.
It is a plant hormone. The name is from Latin salix for willow treeIt is an ingredient in some anti-acne productsSalts and esters of salicylic acid are known as salicylates. The 2D chemical structure image of aspirin is also called skeletal formula which is the standard notation for organic molecules.
The carbon atoms in the chemical structure of aspirin are implied to be located at the corners and hydrogen atoms attached to carbon atoms are not indicated each carbon atom is considered to be associated with enough hydrogen atoms to provide the carbon atom. Acetylsalicylic acid is an acetic acid ester derivative of salicylic acid. The earliest known uses of the drug can be traced back to the Greek physician Hippocrates in the fifth century BC.
He used powder extracted from the bark of willows to treat pain and reduce fever. Salicin the parent of the salicylate drug family was successfully isolated in 1829 from willow bark. Sodium salicylate a.
Aspirin is prepared from the reaction of salicylic acid C 7 H 6 O 3 and acetic anhydride C 4 H 6 O 3 to produce aspirin C 9 H 8 O 4 and acetic acid HC 2 H 3 O 2. The formula for this reaction is C 7 H 6 O 3 C 4 H 6 O 3 C 9 H 8 O 4 HC 2 H 3 O 2. How many grams of salicylic acid are needed to make 1000 1-gram tablets of aspirin.
Assume 100 percent yield Solution. For example in an experiment in which you prepare acetylsalicylic acid aspirin from salicylic acid you know from the balanced equation for aspirin synthesis that the mole ratio between the limiting reactant salicylic acid and the product acetylsalicylic acid is 11. Acid anhydrides are the molecules that are capable of forming acidic solutions in water.
The meaning of anhydride is the removal of water. Acid anhydride is an organic functional group consisting of 2 acyl groups combined by an oxygen atom. The non-metals oxide which are capable of reacting with water are also called Acid anhydrides.
To Learn detail about Acid Anhydride Synthesis Chemical. Arachidonic acid is a long-chain fatty acid that is a C20 polyunsaturated fatty acid having four Z-double bonds at positions 5 8 11 and 14. It has a role as a human metabolite an EC 3111 carboxylesterase inhibitor a Daphnia galeata metabolite and a mouse metabolite.
Complete List of Inorganic Acids. CH3COOH Ethanoic AcidAcetic Acid Vinegar is the chemical compound consisting carboxylic acid. It is widely used as a preservative and for seasoning food and salad.
It is also used for cleaning purposes. Those beauty conscious people may also find this chemical helpful as it is also used in a pedicure. CH3CH2OH Ethanol Alcohol is an.
Chemical Name Trivial Name Formula Melting Point o C Equivalent Weight geq Amide Melting Point o C Diamide Melting Point o C pka 1 pka 2. 2-butynedioic acid acetylenedicarboxylic acid HOOCCCCOOH. 57 - 218 - 221.
2-acetoxybenzoic acid acetylsalicylic acid CH 3 COOC 6 H 4 COOH. 144 - 145 - 355. 4-acetylbenzoic acid—-CH 3 COC 6 H 4 COOH.
List the common names together with the chemical names and formulae of 20 household chemicals. Identify 20 chemicals in everyday household items which is a hint to read the ingredients labels on packets of cleaning materials paints and other containers of substances in your home. Or other similar questions which may ask for more or fewer examples.
C 9 H 8 O 4. Used in various medicines. H 2 O 2.
Hydrogen peroxide Used for personal hygiene. Its a highly corrosive alkali which is used for cleaning unblocking sinks drains and toilets. C 6 H 4 C l2.
The information below refers to products available in the United States that contain aspirin. Acetylsalicylic Acid Ecotrin Aspir 81 Bayer Aspirin Drug classes. Platelet aggregation inhibitors salicylates Aspirin systemic is used in the treatment of.
Chemical Formula C 24 H 40 O 4 Synonyms 3alpha5beta7beta-37-dihydroxycholan-24-oic acid 3α5β7β-37-dihydroxycholan-24-oic acid. The drug decreases the. Felix Hoffmann a German chemist produced a stable form of acetylsalicylic acid more commonly known as aspirin.
Aspirin is a derivative of salicylic acid that is a mild nonnarcotic analgesic useful in the relief of headache and muscle and joint aches. Esterification is a chemical reaction used for making esters. The reaction in which a Carboxylic acid combines with an alcohol in.
A chemical equation with a double headed arrow pointing towards both the reactants and products often denote these reversible reaction situations. For example in human blood excess hydrogen ions H bind to bicarbonate ions HCO 3- forming an equilibrium state with carbonic acid H 2 CO 3. If we added carbonic acid to this system some of.