Add sufficient water 50 mL to cover the pH electrode tip during the titration. Acetic acid also forms miscible mixtures with.

Inforshydrocouk 44 0 1527 882060.
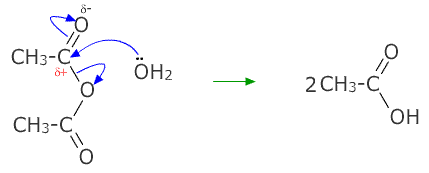
Acetic anhydride and water. Acetic anhydride or ethanoic anhydride is the chemical compound with the formula CH 3 CO 2 O. Commonly abbreviated Ac 2 O it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid which is formed by its reaction with moisture in the air.
Acetic anhydride can be obtained directly by liquid-phase oxidation of acetaldehyde. The peracetic acid formed from oxygen and acetaldehyde reacts under suitable conditions with a second molecule of acetaldehyde to form acetic anhydride and water. Rapid removal of the reaction water and the use of suitable catalysts are essential in this process.
ACETIC ANHYDRIDE reacts violently on contact with water steam methanol ethanol glycerol and boric acid. Reaction with water is particularly dangerous in presence with mineral acids eg nitric perchloric chromic sulfuric acid Chem. Acetic acid ə ˈ s iː t ɪ k systematically named ethanoic acid ˌ ɛ θ ə ˈ n oʊ ɪ k is an acidic colourless liquid and organic compound with the chemical formula CH 3 COOH also written as CH 3 CO 2 H C 2 H 4 O 2 or HC 2 H 3 O 2.
Vinegar is no less than 4 acetic acid by volume making acetic acid the main component of vinegar apart from water and other trace elements. Because acetyl salicylic acid aka Aspirin has an acetate group and acetic anhydride has one that is a good leaving group. The typical lab-ready reaction for this synthesis is.
Basically the acid acts as a catalyst not protonating water though because acetic anhydride is violently reactive with water and the hydroxyl on 2-hydroxybenzoic acid acts as a nucleophile. Hold the large test tube containing the acetic anhydride in cold water to cool the reaction and add the alcohol to it in several increments. Without cooling the strongly exothermic reaction could become too vigorous.
Then place the tube in hot water at about 70º C for 5 min to complete the reaction. Add eight drops of water using a Pasteur pipet. ACETIC ANHYDRIDE CAS Number.
UN 1715 DOT Hazard Class. 8 Corrosive —– HAZARD SUMMARY Acetic Anhydride can affect you when breathed in and may be absorbed through the skin. Acetic Anhydride is a HIGHLY CORROSIVE CHEMICAL and contact can severely irritate and burn the skin and eyes with possible eye damage.
Breathing Acetic Anhydride. The reaction rate is first order with respect to acetic anhydride concentration CA ie. -TA KCA The rate constant k can be determined from the relationship.
K a eb- 2 where k is in the unit of min T is the system temperature in Kelvin scale and a b and care constants. For this reaction a 0158 b 1855 and c 5529. It can be assumed that the content inside the CSTR is mixed.
Identification of the substancemixture and of the supplier Product name. Acetic Anhydride ManufacturerSupplier Trade name. S25119A Recommended uses of the product and restrictions on use.
AquaPhoenix Scientific Inc 9 Barnhart. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
The main target users are workers and those responsible for occupational safety and health. The ICSC project is a common undertaking between the World Health Organization WHO and. 20 grams of salicylic acid 50 mL of acetic anhydride and 5 drops of 85 phosphoric acid solution were placed into a 50 mL Erlenmeyer flask.
A 70-80 C hot water bath was prepared by placing a 250 mL beaker on a hot plate with a thermometer to monitor temperature. The 50 mL Erlenmeyer flask with the mixture of salicylic acid acetic anhydride and phosphoric acid was partially submerged in. Acetic acid CH 3 COOH also called ethanoic acid the most important of the carboxylic acidsA dilute approximately 5 percent by volume solution of acetic acid produced by fermentation and oxidation of natural carbohydrates is called vinegar.
A salt ester or acylal of acetic acid is called acetateIndustrially acetic acid is used in the preparation of metal acetates used in some. Acetic acid or acetic anhydride can explode with nitric acid if not kept cold. Potassium hydroxide residue in a catalyst pot reacted violently when acetic acid was added MCA Case History 920.
During the production of terephthalic acid n-xylene is oxidized in the presence of acetic acid. During these processes detonating mixtures may be produced. Addition of a small amount of water.
When two molecules of acetic acid undergo a condensation reaction together the product formed is acetic anhydride. Acetic Acid as a Solvent. In its liquid state CH 3 COOH is a hydrophile readily dissolves in water and also a polar protic solvent.
A mixture of acetic acid and water is in this manner similar to a mixture of ethanol and water. Acetic acid also forms miscible mixtures with. Calculate the molarity of sodium hydroxide.
Part B Determination of Acetic Acid Concentration in Vinegar. 0 mL of vinegar to a clean dry 150 mL beaker using a 10-mL volumetric pipet. Add sufficient water 50 mL to cover the pH electrode tip during the titration.
Record a titration curve using the MeasureNet pH probe and drop. Acetic Anhydride 73 Acetic Ether See Ethyl Acetate 73 Acetone 120 Acetonitrile Methyl Cyanide NR Acetophenone 73 Acetyl Propane 130 Acetylene 73 Acid Mine Water 150 Acrylic Acid NR Acrylonitrile 73 Adipic Acid Aqueous 120 Alcohol Allyl 140 Alcohol Amyl 73 Alcohol Butyl 180 Alcohol Ethyl 180 Alcohol Isopropyl 120 Alcohol Methyl 180 Alcohol Polyvinyl 120 Allyl Chloride 73 Alum See. Chloride Cl 04 ppm.
Phosphate PO₄ 04 ppm. Heavy metals as Pb 05 ppm. Sulfate SO₄ 04 ppm.
Ag Silver 0005 ppm. Al Aluminium 0020 ppm. Perchloric Acid acetic anhydride bismuth alcohol paper wood oil and grease Peroxides organic acids friction heat sparks Phosphorous white air oxygen alkalis reducing agents Phosphorous pentoxide water Potassium carbon tetrachloride carbon dioxide water Potassium chlorate sulfuric and other acids.
The acid anhydride functional group results when two carboxylic acids combine and lose water anhydride without water. Symmetrical acid anhydrides are named like carboxylic acids except the ending -acid is replaced with -anhydride. This is true for both the IUPAC and Common nomenclature.
Peracetic acid solution purum 40 in acetic acid. 3 Chemical and Physical Properties. Property Name Property Value Reference.
Computed by PubChem 21 PubChem release 20210507 XLogP3-AA-04. Computed by XLogP3 30 PubChem release 20210507 Hydrogen Bond Donor. Glacial acetic acid 100 Acetic acid 80 Acetic anhydride Formic acid 85 Propanoic acid 100 also called Propionic acid Mixtures of acids and flammable liquids Organic Acids.
Butyric acid Pentanoic acid etc. Hydrochloric acid Sulfuric acid Phosphoric acid Hydrofluoric Acid etc Poisons Toxic chemicals Many are in aqueous solution but they are also. Soundspeeds for most commonly used liquids water and sea water.
Inforshydrocouk 44 0 1527 882060. Home Sound Speeds in Water Liquid and Materials. Sound Speeds in Water Liquid and Materials.
Sound Speed or sound velocity refers to the speed of sound waves passing through an elastic medium. The actual speed depends upon the medium for example sound waves. Making esters from alcohols and acyl chlorides.
Esters can also be made from the reactions between alcohols and either acyl chlorides acid chlorides or acid anhydridesIf you add an acyl chloride to an alcohol you get a vigorous even violent reaction at room temperature producing an ester and clouds of steamy acidic fumes of hydrogen chloride. In the first step of this one-carbon homologation the diazomethane carbon is acylated by an acid chloride or mixed anhydride to give an α-diazoketone. The excess diazomethane can be destroyed by addition of small amounts of acetic acid or vigorous stirring.
Most α-diazoketones are stable and can be isolated and purified by column chromatography see recent literature for specific methods. Water Treatment In Perth Australia. All Chemical Manufacturing Consultancy Pty Ltd is one of the successful water filtration equipment and leading chemical suppliers in Perth Australia.
All Chemical have highly experienced professional chemical engineers working at its core. We have been in this industry for more than 15 years which is a testimony to our vast knowledge and impeccable.