
During long-term therapy metabolic acidosis and electrolyte imbalance may occasionally occur. Acetazolamide is a sulfonamide derivative.
R renal tubular acidosis Type I distal.
Acetazolamide and metabolic acidosis. Metabolic acidosis is a serious electrolyte disorder characterized by an imbalance in the bodys acid-base balance. Metabolic acidosis has three main root causes. Increased acid production loss of bicarbonate and a reduced ability of the kidneys to excrete excess acids.
Metabolic acidosis can lead to acidemia which is defined as arterial blood pH that is lower than 735. Metabolic acidosis is primary reduction in bicarbonate. Tubulointerstitial renal disease and when carbonic anhydrase inhibitors eg acetazolamide are taken.
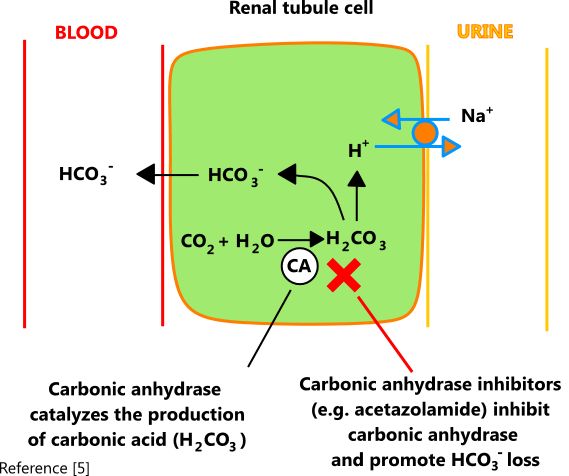
Symptoms and Signs. Symptoms and signs see table Clinical Consequences of Acid-Base Disorders Clinical Consequences of Acid-Base Disorders Acid-base disorders are pathologic changes in carbon dioxide partial pressure Pco2 or. In patients receiving acetazolamide or other carbonic anhydrase inhibitors proximal reabsorption of bicarbonate is decreased resulting in increased distal delivery and HCO3- appears in urine.
This results in a hyperchloraemic metabolic acidosis and is essentially a form of proximal renal tubular acidosis but is usually not classified as such. A metabolic acidosis is an abnormal primary process or condition leading to an increase in fixed acids in the blood - resulting in a fall in arterial plasma bicarbonate. I A gain of strong acid ii A loss of base.
The gain of strong acid may be endogenous eg ketoacids from lipid metabolism or exogenous NH4Cl infusion. Bicarbonate loss may occur. Acetazolamide is a sulfonamide derivative.
Blood disorders rashes and other sulfonamide-related side-effects occur occasionallypatients should be told to report any unusual skin rash. If electrolyte disturbances and metabolic acidosis occur these can be corrected by administering bicarbonate. Metabolic acidosis is characterized by normal or high anion gap situations.
If the primary problem is direct loss of bicarbonate gain of chloride or decreased ammonia production the anion gap is within normal limits. If the primary problem is the accumulation of organic anions such as ketones or lactic acid the condition is known as high anion gap acidosis. Compensatory mechanisms to.
A acetazolamide use. R renal tubular acidosis Type I distal. Type II proximal.
Type IV hyporeninemic hypoaldosteronism. D diarrhea U uretosigmoid fistula because the colon will waste bicarbonate. P pancreatic fistula because of alkali lossthe pancreas secretes a.
Hyperchloremic acidosis is a form of metabolic acidosis associated with a normal anion gap a decrease in plasma bicarbonate concentration and an increase in plasma chloride concentration see anion gap for a fuller explanation. Although plasma anion gap is normal this condition is often associated with an increased urine anion gap due to the kidneys inability to secrete ammonia. Before taking this medicine.
You should not use acetazolamide if you are allergic to it or if you have. Severe liver disease or cirrhosis. An electrolyte imbalance such as acidosis or low levels of potassium or sodium in your blood.
This is especially true in certain forms of metabolic acidosis. For example in high anion gap acidosis secondary to accumulation of organic acids lactate and ketones these anions are eventually metabolized to HCO 3-When the underlying disorder is treated the serum pH corrects. Thus caution should be exercised in these patients when providing alkali to raise the pH much higher than 720.
Metabolic acidosis is usually associated with a reduction in plasma pH although serum concentration of hydrogen ions may be near normal when a mixed acid-base disorder is present. For instance the coexistence of vomiting-induced metabolic alkalosis may contribute to the rise in plasma pH in patients with metabolic acidosis. Common causes of metabolic acidosis include diabetic.
Acetazolamide is a. Acetazolamide can be administered to patients with a metabolic alkalosis to promote retention of hydrogen ions at the level of the renal tubule. For the reduction of Intraocular pressure Acetazolamide inactivates carbonic anhydrase and interferes with the sodium pump which decreases aqueous humor formation and thus lowers IOP.
Acetazolamide increases toxicity of metformin by Other see comment. Decreases serum bicarbonate and induce non-anion gap hyperchloremic metabolic acidosis. Acetazolamide will increase the level or effect of mexiletine by passive renal tubular reabsorption - basic urine.
3 acetazolamide PRN contraction alkalosis. More on this in the section below on contraction alkalosis. Acetazolamide alone is generally sufficient but in severe cases additional therapies may also be needed.
4 potassium-sparing diuretic if available Potassium-sparing diuretics may help prevent hypokalemia hypomagnesemia and metabolic. Loss of appetite electrolyte disturbances metabolic acidosis and hypokalemia with long term therapy hyponatremia osteomalacia with long-term therapy hyperhypoglycemia. Rare 001 to 01.
During long-term therapy metabolic acidosis and electrolyte imbalance may occasionally occur. This can usually be corrected by the administration of bicarbonate. Adverse reactions during short-term therapy are usually non-serious.
This condition invariably subsides upon diminution or withdrawal of the medication. Acetazolamide is a sulphonamide derivative and therefore some. Acetazolamide should not be used to alkalinize urine following salicylate overdose since it may worsen metabolic acidosis.
Acetazolamide is well absorbed from the gastrointestinal tract. Acetazolamide is distributed throughout body tissues. It concentrates principally in erythrocytes plasma and kidneys and to a lesser extent in liver muscles eyes and the central nervous system.
When a metabolic acidosis is suspected it is crucial to calculate the anion gap. This is defined as. Carbonic anhydrase inhibitors such as acetazolamide create a medically induced type 2 proximal renal tubular acidosis scenario by inhibiting bicarbonate reabsorption in the proximal nephron.
Many of the exogenous causes of hyperchloremic acidosis are logical evaluations. Acidosis is a serious metabolic imbalance in which there is an excess of acidic molecules in the body. This can occur as a result of acid overproduction impaired acid transport acid underexcretion or any combination.
With overproduction the body makes too much acid. This can occur in sepsis a life-threatening widespread infection in which the body makes too much lactic acid. Acetazolamide 250 to 375 mg orally or IV once or twice a day increases HCO 3 excretion but may also accelerate urinary losses of K and phosphate PO 4.
Volume-overloaded patients with diuretic-induced metabolic alkalosis and those with posthypercapnic metabolic alkalosis may especially benefit. In patients with severe metabolic alkalosis pH 76 and kidney failure who otherwise. Acetazolamide may interact with cisapride methenamine anticonvulsants other diuretics.
Metabolic acidosis and electrolyte imbalance may occur. Transient myopia has been reported. This condition invariably subsides upon diminution or discontinuance of the medication.
Other occasional adverse reactions include urticaria melena hematuria glycosuria hepatic insufficiency flaccid. Metabolic alkalosis commonly occurs during diuresis. This isnt a contraindication to further diuresis.
Ongoing diuresis may be performed if needed but this must be done with simultaneous treatment of the metabolic alkalosis eg using acetazolamide spironolactone and potassium chloride supplementation. A base excess 3 metabolic alkalosis a base excess -3 metabolic acidosis. The arterial oxygen saturation.
Step by Step ABG Analysis. Step One Assessing pH. Look at pH and determine if it is acidotic 745.
PH is the best overall indicator in determining the acid-base status of the patient. Acetazolamide is used to prevent and reduce the symptoms of altitude sickness. This medication can decrease headache tiredness nausea dizziness and shortness of breath that can occur when you.
Acetazolamide also appears safe and effective in patients with metabolic alkalosis following treatment of respiratory acidosis from exacerbations of chronic obstructive pulmonary disease COPD. 14 15 One randomized trial found that the duration of mechanical ventilation in patients with COPD or obesity-hypoventilation syndrome with metabolic alkalosis was not significantly reduced in.