It is used to stabilize electron ions during electroplating. Cyanide Structure CN.
3739 K Solubility in water.
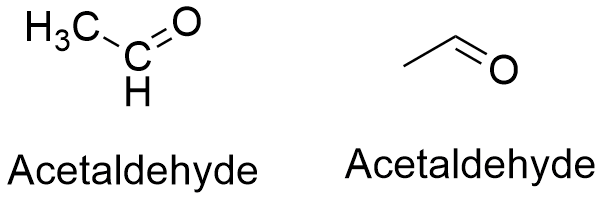
Acetaldehyde molar mass. Use the best molar mass calculator for finding molar mass and molecular weight. Find molecular mass and molecular weight with the online Molecular Weight Calculator. Molecular Weight Calculator Chemical Formula.
Compounds Abietic acid C19H29COOH Acenaphthene C12H10 Acenaphthoquinone C12H6O2 Acenaphthylene C12H8 Acetaldehyde CH3CHO Acetanilide C8H9NO Acetic acid. What is the molar mass of sodium carbonate Na2CO3. Since sodium carbonate contains one carbon atom two sodium atoms and three oxygen atoms the molecular weight is.
230 x 2 46. 120 x 1 12. 16 x 3 48.
If molecular formula calculator add up the total value which is 12 46 48 106. Therefore the molar mass of Na2CO3 is 106 gmol. In the NaOH.
Molecular mass or molar mass are used in stoichiometry calculations in chemistry. In related terms another unit of mass often used is Dalton Da or unified atomic mass unit u when describing atomic masses and molecular masses. It is defined to be 112 of the mass of one atom of carbon-12 and in older works is also abbreviated as amu.
Also important in this field is Avogadros number N. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4.
Atomic mass calculator serves as an online way to find out the masses of atoms of an element present either in a free state or combined form. Using an atomic mass calculator saves you from the hassle of referring to periodic tables again and again or memorizing the atomic mass of each element. 46025 gmol 1 Appearance Colorless fuming liquid Odor.
1220 gmL Melting point. 84 C 471 F. 2815 K Boiling point.
1008 C 2134 F. 3739 K Solubility in water. Miscible with ether acetone ethyl acetate glycerol methanol ethanol Partially soluble in benzene toluene xylenes.
054 Vapor pressure. 44053 gmol Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. At room temperature acetaldehyde H 3 CCOH is more stable than vinyl alcohol by 427 kJmol.
H 2 CCHOH H 3 CCOH ΔH 298g 427 kJmol. The industrial synthesis of acetaldehyde Wacker process proceeds via the intermediacy of a. And weve already calculated their molar masses for you and you see that they have very close molar masses.
And so based on what you see in front of you which of these you think would have a higher boiling point a sample of pure propane or a sample of pure acetaldehyde. Pause this video and think about that. All right well in previous videos when we talked about boiling points and why.
For a gas of constant molar concentration ck. Mass transfer coefficients for the faces of the cylinders are quite different each other and changes drastically as the width to height ratio is varied. Such variations are closely connected with the complex flow phenomena around the rectangular cylinder.
2 The average mass transfer data are in good agreement with the heat transfer. Calculadora de Masa Molar. Common Organic Compounds Elements of the periodic table.
Al calcular el consumo de ciertos productos químicos de RO para el pretratamiento o postratamiento de ósmosis inversa a menudo es necesario convertirlos en moles. Por ejemplo ciertos tipos de generadores de dióxido de cloro ClO 2 usarían hipoclorito de sodio NaOCl. Chemical Reaction Formula Atomic Mass Formula Chemical Formula Enthalpy Formula.
Formula Titanium oxide Formula Chlorine trifluoride Formula Phosphorous trichloride Formula Formaldehyde Formula Acetaldehyde Formula Ethyl acetate Formula Acetone Formula Aspirin Formula Acetaminophen Formula Urea Formula Ethyl alcohol Formula Methane Formula Ethane Formula Propane Formula. Molecular Weight Molar Mass. Cyanide Structure CN.
Cyanide Structure CN. CN Uses Cyanide It is used in the mining of gold. It is used to stabilize electron ions during electroplating.
Cyanide compound such as sodium nitroprusside is used in clinical chemistry. Illegally it is used to capture fish for sea market or aquarium. It is used in the making of.
13445 gmol anhydrous 17048 gmol dihydrate Appearance yellow-brown solid anhydrous blue-green solid dihydrate Odor. 3386 gcm 3 anhydrous 251 gcm 3 dihydrate Melting point. 498 C 928 F.
771 K anhydrous 100 C dehydration of dihydrate Boiling point. 993 C 1819 F. 1266 K anhydrous decomposes Solubility in water.
706 g100 mL. Molar mass gmol Density Range of concentration. C 2 H 4 O.
C 2 H 5 NO. C 3 H 6 O. C 2 H 3 N.
Alcohols also have much higher boiling points than alkanes of the same molecular weight. For example propane molecular mass 4208 gmol has a boiling point of -445C while ethanol MM 4607 gmol has a boiling point of 783C.