
Safety and efficacy of aryl-substituted primary alcohol aldehyde acid ester and acetal derivatives belonging to chemical group 22 when used as flavourings for all animal species View page or View pdf. A second example in which an aldehyde is similarly reduced to a methyl group also illustrates again the use of an acetal protective group.
Drawing aldehyde ketone formulas from names.
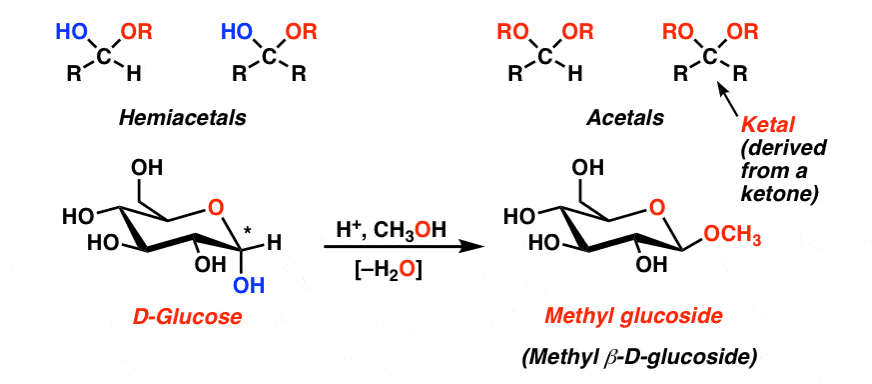
Acetal to aldehyde. Aldehyde to acetal conversion. Ketone to ketal conversion. Acetals are stable compared to hemiacetals but their formation is a reversible equilibrium as with esters.
As a reaction to create an acetal proceeds water must be removed from the reaction mixture for example with a DeanStark apparatus lest it hydrolyse the product back to the hemiacetal. The formation of acetals reduces the. In one example it converts upon treatment with ammonia to 5-ethyl-2-methylpyridine aldehyde-collidine.
Cyclic oligomers of acetaldehyde CH 3 CHO n. Paraldehyde n 3 left and metaldehyde n 4 right Three molecules of acetaldehyde condense to form paraldehyde a cyclic trimer containing C-O single bonds. Similarly condensation of four molecules of.
A second example in which an aldehyde is similarly reduced to a methyl group also illustrates again the use of an acetal protective group. The mechanism of this useful transformation involves tautomerization of the initially formed hydrazone to an azo isomer and will be displayed on pressing the Show Mechanism button. The strongly basic conditions used in this reaction preclude its.
Flavouring Group Evaluation 53 FGE53. Consideration of phenethyl alcohol aldehyde acid and related acetals and esters evaluated by JECFA 59th meeting structurally related to phenethyl alcohol aldehyde esters and related phenylacetic acid esters evaluated by EFSA in FGE14 2005 and one phenoxyethyl ester evaluated in FGE23 2006 Commission Regulation EC No 15652000 of 18. Safety and efficacy of aryl-substituted primary alcohol aldehyde acid ester and acetal derivatives belonging to chemical group 22 when used as flavourings for all animal species View page or View pdf.
EPA Substance Registry Services TSCA. This convenient mild chemoselective method allows acetalization of an aldehyde in the presence of ketone unsymmetrical acetal formation and tolerates acid-sensitive protecting groups. Chem 2002 67 5842-5845.
Pd catalysis enables a highly efficient and simple method for masking a broad range of carbonyl groups as acetals and ketals in. This characteristic makes an acetal an ideal protecting group for aldehyde molecules that must undergo further reactions. A protecting group is a group that is introduced into a molecule to prevent the reaction of a sensitive group while a reaction is carried out at some other site in the molecule.
The protecting group must have the ability to easily react back to the original group from which. Un aldéhyde est un composé organique faisant partie de la famille des composés carbonylés dont lun des atomes de carbone primaire relié au plus à 1 atome de carbone de la chaîne carbonée porte un groupement carbonyle. Un aldéhyde contient donc la séquence.
R représente une chaîne carbonée Laldéhyde le plus simple R réduit au seul atome H est le formaldéhyde ou. 2-Phenylpropionalde-hyde dimethyl acetal. Hydratropic aldehyde dimethyl acetal.
Non-reducing agents dont have free ketone or aldehyde groups and therefore contain an acetal instead of a hemiacetal. An acetal has two O-R groups one R group and a H atom attached to the same carbon. The key difference between an acetal and a hemiactal is that in a hemiacetal an OH group replaces one of the OR acetal groups A sugar without a hemiacetal is non-reducing.
Aldehyde aus neulateinisch alcoholus dehydrogenatus dehydrierter Alkohol oder Alkohol dem Wasserstoff entzogen wurde sind chemische Verbindungen mit der funktionellen Gruppe CHO die Aldehydgruppe oder auch Formylgruppe genannt wird. Die Carbonylgruppe der Aldehyde trägt im Unterschied zu den Ketonen einen Wasserstoff- und einen Kohlenstoffsubstituenten. D is a covalent acetal bond that is stable at neutral pH.
38_____ D-avatose and D-maltose a both have α and β anomers that can mutarotate. B both contain a hemiketal bond in their structures. C both have a potential aldehyde group that can be oxidized.
D both contain one pyranose and one furanose. 39_____ D-avatose and D-sucrose. Aldehyde C-H at 2700 2900 CO at 1730 N H amine and alkene C-N bands in the fingerprint region CC bands near 1650 N nitrile C N at 2250 OH alcohol and aromatic ring O-H at 3500 O ketone and alkene CO at 1720 O O OH O ether C-O bands in the fingerprint region actually a special kind of ether-like functionalgroup called an acetal carboxylic acid O-H at 2500-3200 CO at 1720.
Welcome to Best Value Chem. As a leading creator and manufacturer of flavors and fragrances Best Value Chem P Limited is dedicated to excellence in every area of our business using knowledge creativity innovation and technology to provide our customers with superior consumer understanding and the highest quality products and service. Nomenclature On appelle alcool un composé dans lequel un groupe caractéristique hydroxyle-OH est lié à un atome de carbone saturé.
La chaîne principale est la chaîne la plus longue qui porte le groupe -OHLa numérotation de la chaîne est choisie de façon que le groupe -OH ait le numéro le plus petitLe nom de lalcool est formé en ajoutant le suffixe. The enophile can also be an aldehyde ketone or imine in which case β-hydroxy- or β-aminoolefins are obtained. These compounds may be unstable under the reaction conditions so that at elevated temperature 400C the reverse reaction takes place - the Retro-Ene Reaction.
While mechanistically different the Ene reaction can produce a result similar to the Prins Reaction. Mechanism of the. Un ose réducteur est un type dose possédant un groupe aldéhyde ou capable den former un par isomérieLa forme hémiacétal cyclique dun aldose peut souvrir pour présenter une fonction aldéhyde et certains cétoses peuvent par tautomérie se transformer en aldoses.
Cependant les acétals notamment ceux formant des liaisons polyosidiques ne peuvent pas facilement devenir des. 2-Nonyn-1-al dimethyl acetal. 1335-46-2 127-42-4 127-43-5 127-51-5 7779-30-8 79-89-0 1335-94-0.
Methyl ionone mixed isomers. The carbonyl at C1 is an aldehyde. Aldose the carbonyl at any other carbon is a ketone.
Number of carbons three carbons. Cyclic form Chapter 236 and 237 CHO HOH HO H H OH HOH CH2OH CH2OH O HO H H OH H OH CH2OH glucose hexose. Wird ein weiterer Alkohol addiert entsteht ein Voll-Acetal.
Sowohl Aldehyde als auch Ketone bilden mit Alkoholen Halbacetale letztere bezeichnet man auch als Halbketale. Aufgrund günstiger Positon kann sich auch eine Hydroxygruppe desselben Moleküls an die Carbonyl-Gruppe CO addieren. Diese Situation kann man bei Pentosen und Hexosen beobachten die in einer.
Drawing aldehyde ketone formulas from names. Drawing carboxylic acid derivative formulas. Drawing carboxylic derivatives formulas from names.
Identifying Stereoisomers of Substituted Cycloalkanes. Les disaccharides sont lun des quatre groupes chimiques de glucides monosaccharides disaccharides oligosaccharides et polysaccharidesLes types les plus communs de disaccharides - saccharose lactose et maltose - ont douze atomes de carbone avec la formule générale C 12 H 22 O 11Les différences dans ces disaccharides sont dues à des arrangements atomiques. Imine and Enamine Hydrolysis Mechanism.
Reaction of Aldehydes and Ketones with CN Cyanohydrin Formation. Hydrolysis of Acetals Imines and Enamines-Practice Problems. The Wittig Reaction-Practice Problems.
Carboxylic Acids and Their Derivatives-Nucleophilic Acyl Substitution. Preparation of Carboxylic Acids.