Monosaccharides do not yield smaller molecular weight sugars on hydrolysis. It was noted that exogenous toxins were the most common cause of rhabdomyolysis with illicit drugs alcohol and prescribed drugs responsible for 46 of rhabdomyolysis in hospitalized patients.
Temperature is measured using.
Absolute alcohol molecular weight. Determine the absolute viscosity of Polymer solutions of different concentrations. Determine the viscosity average molecular weight of a polymer. Viscosity is an internal property of a fluid that offers resistance to flow.
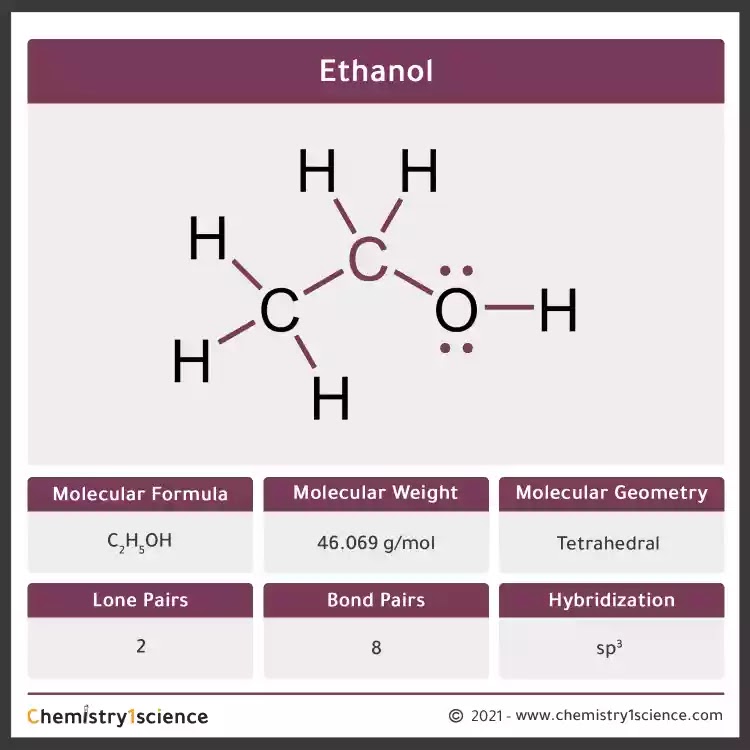
It is due to the internal friction of molecules and mainly depends on the nature temperature of the liquid. Many methods are available for measuring viscosity of. Ethanol also called ethyl alcohol grain alcohol drinking alcohol or simply alcohol is an organic chemical compoundIt is a simple alcohol with the chemical formula C 2 H 6 O.
Its formula can be also written as CH 3 CH 2 OH or C 2 H 5 OH an ethyl group linked to a hydroxyl group and is often abbreviated as EtOHEthanol is a volatile flammable colorless liquid with a. For nucleic acid purification and precipitation. Ultrapure molecular biology grade ethanol is used for the purification and precipitation of biomolecules such as nucleic acids and proteins.
It is also used in histology to prepare staining and destaining reagents and for dehydrating. In the second phase aromatic oils are extracted and separated from most of the plant waxes and non-aromatic materials as the concrète is washed in ethyl alcohol followed by filtration andor centrifuging. The alcohol is recovered by gentle vacuum.
The remaining aromatic material is called an absolute. Absolutes are the most concentrated form of botanical fragrance highly regarded in. Ethanol is a primary alcohol that is ethane in which one of the hydrogens is substituted by a hydroxy group.
It has a role as an antiseptic drug a polar solvent a neurotoxin a central nervous system depressant a teratogenic agent a NMDA receptor antagonist a protein kinase C agonist a disinfectant a human. Air - Molecular Weight and Composition - Dry air is a mixture of gases where the average molecular weight or molar mass can be calculated by adding the weight of each component. Benzene Gas - Specific Heat - Specific heat of Benzene Gas - C6H6 - at temperatures ranging 250 - 900 K.
The ABSOLUTE ZERO the lowest temperature wich can be obtained. Temperature ºC absolute zero -27316 melting ice 00 human body 37 filament of electric bulb 2 500 surface of the sun 6 000 Interior of the sun 15 000 000 Note that the sizes of the degree Celsius and degree Kelvin are the same and therefore temperature differences are the same on both scales. Temperature is measured using.
LFQSCWFLJHTTHZ-UHFFFAOYSA-N Copy CAS Registry Number. This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. Since mass is a very specific property of a molecule determination of the molecular weight with high precision allows to solve many problems in proteomics.
Over the past twenty years ESI-mass spectrometry has emerged as a powerful tool in the life science to determine the identity 220 222 quantity 223 224 and structural properties 6 225 227 of the protein molecules. Alcohols from these distillations are methyl alcohol ethanol propanol butanol iso-butanol iso-amyl alcohol etc. Most of the alcohols are used by the flavour and fragrances industry as raw materials which eventually go into the beverages food and some daily products.
According to the US and EU regulations about natural ingredients materials from fermentation are regarded as natural. Here c is the concentration of ethanol V is the final volume of the sample M W is the molecular weight of ethanol gmol ε is the extraction coefficient of NADH at 340 nm ε 6300 1 mol 1 cm 1 d is the light path cm v is the sample volume mL. It is a simple alcohol with a molecular formula of C 2 H 6 O and a molecular weight of 460684 gmol.
The molecular formula of ethanol may also be written as CH 3 CH 2 OH or as C 2 H 5 OH. The latter can also be thought of as an ethyl group linked to a hydroxyl alcohol group and can be abbreviated as EtOH. Ethanol also called ethyl alcohol grain alcohol or alcohol a member of a class of organic compounds that are given the general name alcohols.
Its molecular formula is C 2 H 5 OH. Ethanol is an important industrial chemical. It is used as a solvent in the synthesis of other organic chemicals and as an additive to automotive gasoline forming a mixture known as a gasohol.
Answer 1 of 5. Density is mass per unit volume. Weight is the force of gravity which is mass times acceleration.
Fmg where g is the acceleration due to gravity 98 ms2. Since mρV weight equals ρVg. C 2 H 6 O.
Names and Identifiers Properties Safety and Handling NMR Spectrum Synthesis Route Precursor and Product Computational chemical data 429 Suppliers. SAFETY DATA SHEETS According to Globally Harmonized System of Classification and Labelling of Chemicals GHS - Sixth revised edition. The simple sugars or monosaccharides that are nutritionally important are six-carbon compounds exhibiting alcohol.
Monosaccharides do not yield smaller molecular weight sugars on hydrolysis. Many simple sugars occur in woody plants but usually in very small amounts probably because of their rapid incorporation in polysaccharides. Exceptions are the six-carbon sugars glucose and fructose.
Ethanol Ethyl Alcohol C 2 H 5 OH is a volatile flammable colorless liquid with a slight characteristic odorIt is produced via petrochemical processes or naturally by the fermentation of sugars by yeasts. Ethanol is most commonly consumed as a popular recreational drugIt is a psychoactive substance and is the principal type of alcohol found in alcoholic drinks. Rhabdomyolysis may be seen commonly with AKI due to many toxins including alcohol heroin cocaine and synthetic cannabinoids.
Multiple toxins may be involved in causing rhabdomyolysis. It was noted that exogenous toxins were the most common cause of rhabdomyolysis with illicit drugs alcohol and prescribed drugs responsible for 46 of rhabdomyolysis in hospitalized patients. The axes are based on how many alcohol molecules there are per hundred molecules rather than on a weight basis This is because one alcohol molecule evaporates for every water molecule that condenses.
Thus the number of molecules of vapor passing a given point per second doesnt change as you move up the column and the same goes for the liquid. So if the stripper has for instance four. A Laboratory Manual Third Edition.
A Laboratory Manual Third Edition 1982. Full PDF Package Download Full PDF Package. A short summary of this paper.
36 Full PDFs related to this paper. Download Full PDF Package. Benzyl alcohol appears as a clear colorless liquid with a pleasant odor.
Slightly denser than water. Contact may irritate skin eyes and mucous membranes. May be slightly toxic by ingestion.
Used to make other chemicals. Benzyl alcohol is an aromatic alcohol. Claudia Ojeda-Granados Paolo Abondio Alice Setti Stefania Sarno Guido Alberto Gnecchi-Ruscone Eduardo González-Orozco Sara De Fanti Andres Jiménez-Kaufmann Héctor Rangel-Villalobos Andrés Moreno-Estrada Marco Sazzini Dietary Cultural and Pathogens-Related Selective Pressures Shaped Differential Adaptive Evolution among Native Mexican Populations Molecular Biology and.
Introduction A vast body of evidence from human studies and animal research clearly indicates that chronic heavy alcohol consumption causes structural damage andor disrupts normal organ function in virtually every tissue of the body. In heavy consumers of alcohol the liver is especially susceptible to alcohol-induced injury12 Additionally several other organsincluding the. In terms of the OPW the LLR in effect sets an absolute upper limit for the casting speed.
It has been argued that a principle lower limit is given by the retraction velocity of a meniscus of the pure evaporating solvent 20 22 since below this speed an oversupply of solute may lead to crystal nucleation and growth prior to deposition and hence loss of directionality.