Low heat values are calculated from high heat value test data. The LCC has five carbon atoms and so the parent compound is pentane rule 1.

97377 amu Calculate the average atomic mass for the three isotopes of Silicon.
2 methylpentane molecular mass. 3-Ethyl-2-methylpentane C8H18 CID 11863 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. Sigma-Aldrich Material Safety Data Sheet for 2-methylpentane.
Version 44 February 20 2012. Available from as of November 7 2013. Version 44 February 20 2012.
It is an instrument in which the substances in gaseous or vapor state is bombarded with a beam of electrons to form positively charged ions cations which are further sorted according to their mass to charge ratio to record their masses and relative abundances. It is a sorted collection of the masses of all the charged molecular fragments produced. The molecular weight of a substance also called the molar mass M is the mass of 1 mole of that substance given in M gram.
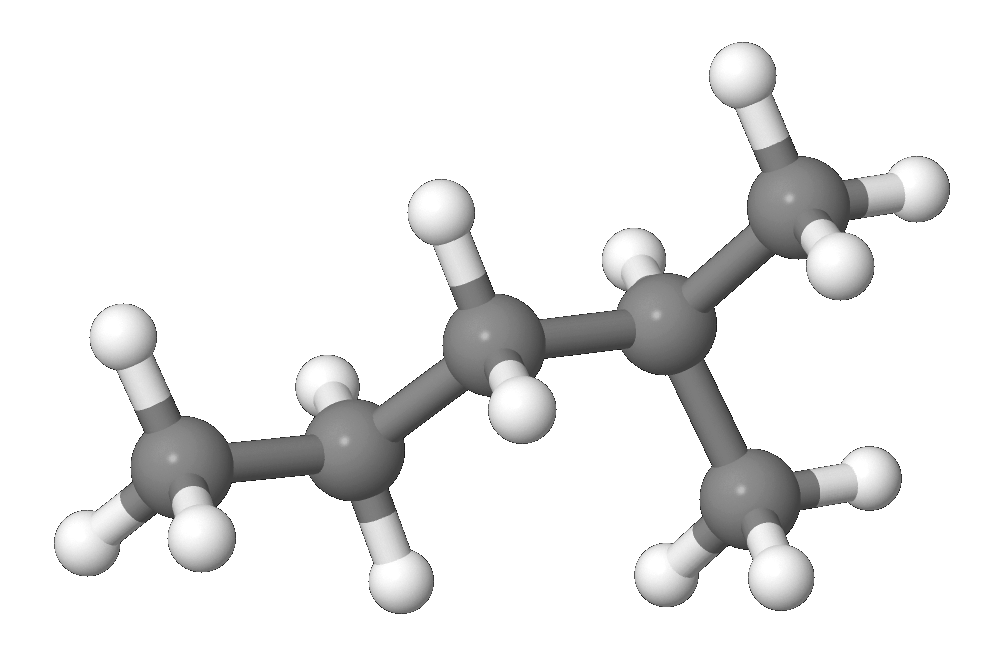
In the SI system the unit of M is kgkmol and in the English system the unit is lblbmol while in the cgs system the unit of M is gmol. 3-Methylpentane is a branched alkane with the molecular formula C 6 H 14. It is a structural isomer of hexane composed of a methyl group bonded to the third carbon atom in a pentane chain.
It is of similar structure to the isomeric 2-methylpentane which has the methyl group located on the second carbon of the pentane chain. Mole-Mass and Mass-Mass Calculations. The Mole in Chemical Reactions.
The Ideal Gas Law and Some Applications. Kinetic Molecular Theory of Gases. Molecular Effusion and Diffusion.
Energyvolume of the fuel. There are two kinds of enthalpy of combustion called higher and lower heating value depending on how much the products are allowed to cool and whether compounds like H 2 O are allowed to condense. The high heat values are conventionally measured with a bomb calorimeter.
Low heat values are calculated from high heat value test data. A C 60 molecule is nonpolar but its molar mass is 720 gmol much greater than that to create an homogeneous solution we need to pick a solvent that has similar inter molecular forces to that of our substance. Water is a solvent for polar molecules and the most common solvent used by living things.
All the ions and proteins in a cell are Aug 21 2021 Even large compounds like hexane. Draw isomers for the following molecular formulas a C 6 H 14 5 isomers b C 5 H 12 3 isomers c C 7 H 16 d C 5 H 10 e C 4 H 8 f C 7 H 14 Question 4. Biologically important reactions p.
LESSON PLAN 22 - Glencoe isomers formed one is termed the endo-product and the other is termed the exo-product. 97377 amu Calculate the average atomic mass for the three isotopes of Silicon. The loss of C 3 H 7 gives a peak mass of 59 and the loss of C 2 H 5 gives a peak mass of 73.
Cyclopentene Of The Best Naming Alkanes Worksheet 1 - Goal keeping Naming Alkanes Worksheet 2. The meso form is a diastereomer of the other forms. The test tube in which a brisk effervescence takes place with the liberation of a colourless gas CO2 is carboxylic acid.
This proposed that these are. The LCC has five carbon atoms and so the parent compound is pentane rule 1. There is a methyl group rule 2 attached to the second carbon atom of the pentane chain.
The name is therefore 2-methylpentane. The LCC has six carbon atoms so the parent compound is hexane rule 1. Methyl groups rule 2 are attached to the second and fifth.
Thermophysical Properties of Fluid Systems. Accurate thermophysical properties are available for several fluids. These data include the following.
Gases - Densities - Densities and molecular weights of common gases like acetylene air methane nitrogen oxygen and others. Gases - Dynamic Viscosity - Absolute viscosities of gases. Hydrogen - Specific Heat - Specific heat of Hydrogen Gas - H 2 - at temperatures ranging 175 - 6000 K.
Academiaedu is a platform for academics to share research papers. For properties that require the molar mass molecular weight for the conversion process the value of the molar mass must be included as the sixth input to the REFPROP routine. Instructions for Use of this Workbook and Converting 9x to 10 Exergy CExergy Flow exergy Closed system exergy 100001 1000 100070 1000 DREFPROPVBREFPROPXLS.
Method validation applies to the technical hexane. 2-methylpentane 3-methylpentane cyclohexane and methylcyclopentane the signal-to-noise SN as well as the limit of quantification LoQ values was found to be 21820 22145 74637 9737 and 085 084 025 193 mg kg 1 respectively. Good linearity repeatability and low carryover of this method have provided an alternative way.
Academiaedu is a platform for academics to share research papers. Polystyrene of a molecular weight Mn not exceeding 5000. Crystalline polystyrene with a melting point of 268 C or more but not more than 272 C and a setting point of 232 C or more but not more than 242 C whether or not containing additives and filling material.
Hydroxyl radicals are highly reactive species that attack most of the organic molecules. They are highly oxidizing in nature which is attributed to their oxidation potential Tchobanoglous et al 2003In addition owing to their nonselective nature many susceptible organic molecules can easily be removed or degraded using hydroxyl radical eg acids alcohols aldehyde aromatics amines. Write balanced equations for the complete combustion of the following hydrocarbons.
Reaction mass of 2-chloroethyl chloropropyl 2-chloroethylphosphonate mixture reaction mass of isomers and 2-chloroethyl chloropropyl 2-chloropropylphosphonate reaction mass of isomers 401-740-0 015-144-00-X reaction mass of pentyl methylphosphinate and 2-methylbutyl methylphosphinate 402-090-0 87025-52-3 015-145-00-5. The molecular mass of an organic acid was determined by the study of its barium salt. 2562 g of salt was quantitatively converted to free acid by.
Write balanced equations for the complete combustion of the following hydrocarbons. Mass of the compound 03780 g Mass of silver chloride 05740 g. In an estimation of sulphur by Carius method 0468 of an organic sulphur compound gave 0668 g of barium sulphate.
Find the percentage of sulphur in the compound. Mass of the compound 0468 g Mass of barium sulphate 0668 g.