
Academiaedu is a platform for academics to share research papers. The molecular weight of a substance also called the molar mass M is the mass of 1 mole of that substance given in M gram.
Three monomers mentioned in this chapter are.
2 methylhexane molecular mass. Property Name Property Value Reference. Computed by PubChem 21 PubChem release 20210507 XLogP3-AA. Computed by XLogP3 30.
Property Name Property Value Reference. Computed by PubChem 21 PubChem release 20210507 XLogP3-AA. Computed by XLogP3 30.
The molecular weight of a substance also called the molar mass M is the mass of 1 mole of that substance given in M gram. In the SI system the unit of M is kgkmol and in the English system the unit is lblbmol while in the cgs system the unit of M is gmol. Nicotine Salts Nicotine Salts Base PG Nicotine Salts Base.
VG Nicotine Salts Base. The loss of C 3 H 7 gives a peak mass of 59 and the loss of C 2 H 5 gives a peak mass of 73. Cyclopentene Of The Best Naming Alkanes Worksheet 1 - Goal keeping Naming Alkanes Worksheet 2.
The meso form is a diastereomer of the other forms. The test tube in which a brisk effervescence takes place with the liberation of a colourless gas CO2 is carboxylic acid. This proposed that these are.
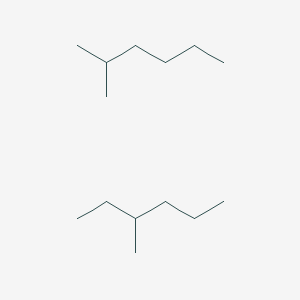
3-Methylpentane is a branched alkane with the molecular formula C 6 H 14. It is a structural isomer of hexane composed of a methyl group bonded to the third carbon atom in a pentane chain. It is of similar structure to the isomeric 2-methylpentane which has the methyl group located on the second carbon of the pentane chain.
Draw isomers for the following molecular formulas a C 6 H 14 5 isomers b C 5 H 12 3 isomers c C 7 H 16 d C 5 H 10 e C 4 H 8 f C 7 H 14 Question 4. Biologically important reactions p. LESSON PLAN 22 - Glencoe isomers formed one is termed the endo-product and the other is termed the exo-product.
97377 amu Calculate the average atomic mass for the three isotopes of Silicon. Gases - Densities - Densities and molecular weights of common gases like acetylene air methane nitrogen oxygen and others. Gases - Dynamic Viscosity - Absolute viscosities of gases.
Hydrogen - Specific Heat - Specific heat of Hydrogen Gas - H 2 - at temperatures ranging 175 - 6000 K. Alkanes of low molar massthose with from 1 to approximately 10 or so carbon atomsare gases or light liquids that act as anesthetics. Inhaling sniffing these hydrocarbons in gasoline or aerosol propellants for their intoxicating effect is a major health problem that can lead to liver kidney or brain damage or to immediate death by asphyxiation by excluding oxygen.
CHEMISTRY COMPUTING FORMULA MASS WORKSHEET Problem Set-up example. Find the formula mass of CaNO32 Ca. A molecular compound is a group of atoms held together by a covalent bond.
Indicate the number of atoms of each element in the compound by using the following Greek prefixes. Naming ionic compounds worksheet answers chemistry. Academiaedu is a platform for academics to share research papers.
The correct IUPAC name for 2-isopropylbutane is. A 2-ethylpentane b 23-dimethylpentane c 2-methylhexane d 3-methylhexane View Answer Identify each. 1275 Monomers are small molecules with low molecular mass that are joined together to form polymers.
Three monomers mentioned in this chapter are. If a dicarboxylic acid and a dialcohol are combined there is the potential for propagation of the polymer chain at both ends of both monomers. 1283 Flexibility of molecular chains causes flexibility of the bulk polymer.
Chapter 4 review worksheet chemistry answer key. Academiaedu is a platform for academics to share research papers. Chapter 4 section 2 the structure of atoms interactive reader answers.
The empirical formula is CH2O 30 amu. Molecular mass 150 amu. Molecular mass 150 amu 5.
Therefore empirical formula mass 30 amu molecular formula 5 x empirical formula C5 x 1H5 x 2O5 x 1 C5H10O5 327 a Assume a 1000 g sample. From the percent composition data a 1000 g sample contains 2186 g H and 7814 g B. 1 mol H 21.
Reaction mass of 2-chloroethyl chloropropyl 2-chloroethylphosphonate mixture reaction mass of isomers and 2-chloroethyl chloropropyl 2-chloropropylphosphonate reaction mass of isomers 401-740-0 015-144-00-X reaction mass of pentyl methylphosphinate and 2-methylbutyl methylphosphinate 402-090-0 87025-52-3 015-145-00-5. 2-Ethylhexyl acrylate-ethyl acrylate copolymers prepared by copolymerization of 2-ethylhexyl acrylate and ethyl acrylate in a 73 weight ratio and having a number average molecular weight range of 5800 to 6500 and a refractive index n D25 40 percent in 224-trimethyl pentane of 14130-14190. For use as a modifier for nylon resins complying with 1771500 of this chapter and for.
Which of the following is the correct condensed formula corresponding to the carbon-skeleton structure shown. The longest continuous C chain in the structure contains 6 carbon atoms. There are two CH3 groups attached to adjacent carbon atoms of the parent chain.
Structure A is correct. In a carbon-skeleton or line-bond structure for an organic compound. The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements with all substances in their standard statesThe standard pressure value p 10 5 Pa 100 kPa 1 bar is recommended by IUPAC although prior to 1982 the value 100 atm 101325 kPa was used.
Predict the major organic product of the reaction sequence. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Our purpose is to solve the toughest problems in life science by collaborating with the global scientific community and through that we aim to accelerate access to better health for people everywhere.