Alcohols that react with the Lucas reagent are converted to the corresponding alkyl chlorides RCl. Die reine Substanz ist eine bei Raumtemperatur farblose leicht entzündliche Flüssigkeit mit einem brennenden Geschmack und einem charakteristischen würzigen süßlichen Geruch.
However the structure on the left rotates plane-polarized light counter-clockwise.
2 butanol structure. 3-methyl-2-butanol is a secondary alcohol that is 2-butanol carrying an additional methyl substituent at position 3. It has a role as a polar solvent and a plant metabolite. 2-Butanol C4H10O CID 6568 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. In this 3D projection of 2-butanol the structure on the left has the R-configuration while its mirror image on the right is the S-isomer according to the Cahn-Ingold-Prelog rules. However the structure on the left rotates plane-polarized light counter-clockwise.
It is designated as - or l while the S-isomer is or l. Cyclohexanol is the organic compound with the formula HOCHCH 2 5The molecule is related to cyclohexane by replacement of one hydrogen atom by a hydroxyl group. This compound exists as a deliquescent colorless solid with a camphor-like odor which when very pure melts near room temperature.
Billions of kilograms are produced annually mainly as a precursor to nylon. C 4 H 6. C 6 H 6.
MOs and Electrostatic Potential. From the structure above remove all the carbon atoms. Structural formula of butane minus carbons Join all the lines to complete the skeletal formula.
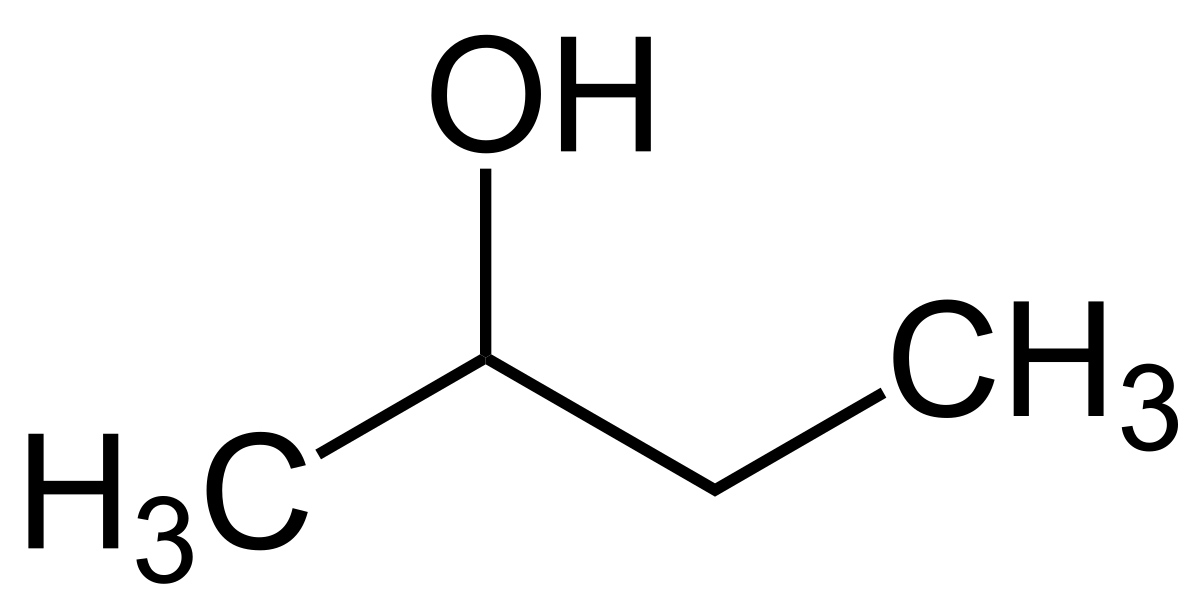
Skeletal formula of butane Example 2. Draw the skeletal formula for butan-2-ol 2-butanol molecular formula C 4 H 10 O given the 2-dimensional full display structural formula below. Structural formula of butanol Identify the carbon skeleton.
1-butanol a primary alcohol 2-butanol a secondary alcohol 2-methyl-2-propanol a tertiary alcohol and phenol are the four known alcohols. 1-propanol 2-propanol 2-methyl-2-butanol and para-chlorophenol are the four possible unknown alcohols. Propanol is used as a solvent or to make other solvents including antifreezes lacquer formulas soaps dye solutions and window.
Draw the structure for each compound. The ending -ol indicates an alcohol the OH functional group and the hex- stem tells us that there are six carbon atoms in the LCC. We start by drawing a chain of six carbon atoms.
The 2 indicates that the OH group is attached to the second carbon atom. Finally we add enough hydrogen. Marking criteriaNasienriglyne Whole structure correctHele struktuur korrek.
2 2 Only functional group correct Slegs funksionele groep korrek Max. Any correct structure of. To see a more detailed description of these isomers see Amyl alcohol.
The molecular formula C 5 H 12 O molar mass. 8815 gmol exact mass. 88088815 may refer to.
The molecular formula C 5 H 12 O may also refer to. CH 3-O-C 4 H 9 that is Methoxy butane. A ketone is a functional group that consists of a carbonyl carbon which is a carbon atom bound to an oxygen atom by a double bond and two alkyl or aryl groups.
Ethanol auch Äthanol Trivialname Alkohol ist ein aliphatischer einwertiger Alkohol mit der Summenformel C 2 H 6 O. Die reine Substanz ist eine bei Raumtemperatur farblose leicht entzündliche Flüssigkeit mit einem brennenden Geschmack und einem charakteristischen würzigen süßlichen Geruch. Bekannt ist Ethanol als Bestandteil von Genussmitteln und alkoholischen Getränken wie Wein.
An acid dissociation constant K a is a quantitative measure of the strength of an acid in solutionIt is the equilibrium constant for a chemical reaction known as dissociation of acidbase reactions. In aqueous solution the equilibrium of acid dissociation can be written symbolically as. HA H 2 O A-H 3 O.
Where HA is an acid that dissociates into A known as the conjugate. CeO2 is Fluorite structured and crystallizes in the cubic Fm-3m space group. The structure is three-dimensional.
Ce4 is bonded in a body-centered cubic geometry to eight equivalent O2- atoms. All CeO bond lengths are 237 Å. O2- is bonded to four equivalent Ce4 atoms to form a mixture of edge and corner-sharing OCe4 tetrahedra.
Alcohol - alcohol - Physical properties of alcohols. Most of the common alcohols are colourless liquids at room temperature. Methyl alcohol ethyl alcohol and isopropyl alcohol are free-flowing liquids with fruity odours.
The higher alcoholsthose containing 4 to 10 carbon atomsare somewhat viscous or oily and they have heavier fruity odours. 2-butanol is a clear organic secondary alcohol. That means the hydroxyl group is attached to the second carbon atom.
The difference in the R-2-butanol and the S-2-butanol has to do with the. Draw the structure of the product formed when the following compound is heated in aqueous base. CH 3 CH 2 CH 2 CH 2 OH ii 2-butanol iii 2-methyl-l-propanol Delhi 2012 Answer.
A State the formulas of the products E and F formed in the following reaction. Thank you for your participation. Your assessment is very important for improving the workof artificial.
Varies with the structure of the alcohol. The Lucas reagent is a solution of ZnCl2 in concentrated HCl. Alcohols that react with the Lucas reagent are converted to the corresponding alkyl chlorides RCl.
To perform a Lucas test place 4-5 drops of ROH in a test tube then add 2 ml of the Lucas reagent stopper the tube and shake. If a reaction occurs the RCl will separate as a distinct. The films consist of densely packed Au nanofibers with a length of 800 nm vertically grown on the surface of the silica-containing wafer Figure 1AThe wide-angle powder X-ray diffraction PXRD patterns of the R-CNAFs Figure 1B show identical reflections indexed as the typical face-centered cubic fcc structure of Au with a space group of F m 3 m JCPDS 04-0784.
If we mentally insert an oxygen atom into a C-H bond we will have an alcohol either 1-butanol or 2-butanol. Both compounds have zero degrees of unsaturation no multiple bonds or rings. If the oxygen atom were inserted in a C-C bond of n-butane ethers would be formed both of which diethyl etherand methyl n-propyl ether have zero degrees of unsaturation.
Thus ignore oxygen atoms in a. 5 free GRE sentence equivalence practice tests with explanations. Our tests contain over 50 sentence equivalence questions to help you prepare for the GRE.
The specific rotation of pure R-2-butanol is -135. What of a mixture of the two enantiomeric forms is S-2-butanol if the specific rotation of this mixture is 54. A 40B 30C 60D 70E None of the above The answer is B since ee.
Is 40 favoring R. 5413540 H CH3 Cl H CH3 H OH H OH CH3 CH3 HO H H OH CH3. Which of the following alkyl halides would.
Solvent Other Names Structure. Class Acetic acid Ethanoic acid CH 3 COOH Calss 3 Acetone 2-Propanone CH 3 COCH 3. 2-Butanol sec-Buty al clohol.
There is no structure for this substance. PMN. SRS Web Services.
Products Building Blocks Explorer Technical Documents Site Content Papers Genes. Keyword MSDS Showing 1-2 of 2 results for MSDS within Products. Sigma-Aldrich SDS Subscription.
Custom and intranet version quarterly.